What Element Has The Largest Ionization Energy
Muz Play
Mar 25, 2025 · 5 min read
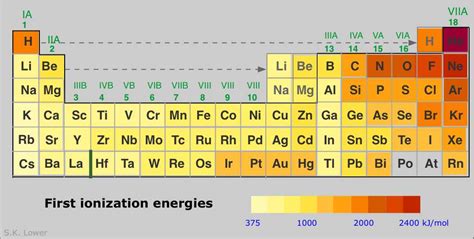
Table of Contents
What Element Has the Largest Ionization Energy? Uncovering the Secrets of Helium
The quest to identify the element boasting the highest ionization energy is a journey into the heart of atomic structure and the forces that govern electron behavior. Ionization energy, a fundamental concept in chemistry and physics, represents the minimum energy required to remove the most loosely bound electron from a neutral gaseous atom or ion. While seemingly simple, this seemingly simple concept unveils a fascinating interplay of nuclear charge, electron shielding, and atomic radius, all culminating in the remarkable properties of specific elements. This article delves deep into the intricacies of ionization energy, exploring its trends across the periodic table and ultimately revealing which element reigns supreme.
Understanding Ionization Energy: A Deeper Dive
Before we pinpoint the champion of ionization energy, let's solidify our understanding of this crucial atomic property. The ionization energy (IE) is expressed in units of kilojoules per mole (kJ/mol) or electron volts (eV). The process of ionization is always endothermic, meaning it requires energy input to overcome the electrostatic attraction between the negatively charged electrons and the positively charged nucleus.
The first ionization energy (IE₁) refers to the energy needed to remove the first electron from a neutral atom:
X(g) → X⁺(g) + e⁻
Subsequent ionization energies (IE₂, IE₃, etc.) represent the energy needed to remove successive electrons from the increasingly positively charged ion. Each subsequent ionization energy is always greater than the previous one because removing an electron leaves a more positive ion, leading to a stronger electrostatic attraction for the remaining electrons.
Periodic Trends: A Map to Ionization Energy
The periodic table provides a powerful framework for understanding the trends in ionization energy. Several key factors influence this property:
1. Effective Nuclear Charge (Z<sub>eff</sub>):
The effective nuclear charge represents the net positive charge experienced by an electron after accounting for the shielding effect of other electrons. A higher Z<sub>eff</sub> leads to a stronger attraction between the nucleus and the outermost electrons, resulting in a higher ionization energy. Moving across a period (from left to right), Z<sub>eff</sub> increases, causing a general increase in ionization energy.
2. Atomic Radius:
The atomic radius, or the size of an atom, plays a crucial role. Smaller atoms have a shorter distance between the nucleus and the outermost electrons, leading to a stronger electrostatic attraction and higher ionization energy. Moving across a period, atomic radius generally decreases, again contributing to the increase in ionization energy.
3. Electron Shielding:
Inner electrons shield the outer electrons from the full positive charge of the nucleus. This shielding effect reduces Z<sub>eff</sub> and consequently lowers the ionization energy. Down a group (from top to bottom), the number of inner electrons increases, enhancing the shielding effect and leading to a decrease in ionization energy.
4. Electron Configuration:
The specific electron configuration of an element significantly influences its ionization energy. Elements with filled or half-filled subshells (like noble gases or elements with a half-filled p-subshell) exhibit higher ionization energies due to enhanced stability. Removing an electron from these stable configurations requires more energy.
The Contenders: Exploring High Ionization Energy Elements
While ionization energy generally increases across a period and decreases down a group, several elements stand out due to their exceptionally high ionization energies. These high values highlight the exceptional stability of their electron configurations.
Helium (He): The Undisputed Champion
Helium (He), with its electron configuration of 1s², emerges as the undisputed champion of ionization energy. Its compact size and the strong attraction between the nucleus and its two electrons contribute to its exceptionally high first ionization energy. Helium's electrons occupy the 1s orbital, which is closest to the nucleus and experiences minimal shielding. This combination of factors makes it incredibly difficult to remove an electron from a helium atom.
Other Notable Elements:
While helium holds the crown, other elements display remarkably high ionization energies:
-
Neon (Ne): A noble gas with a completely filled electron shell (1s²2s²2p⁶), neon exhibits a significantly high ionization energy due to its stable octet configuration.
-
Argon (Ar): Another noble gas, argon shares a similar stability with a filled valence shell (3s²3p⁶), leading to a high ionization energy.
-
Hydrogen (H): While its ionization energy is lower than the noble gases, hydrogen’s single electron is strongly attracted to its single proton, resulting in a relatively high ionization energy.
It is important to note that while helium demonstrates the highest first ionization energy, subsequent ionization energies for all elements increase significantly. This increase stems from the progressively stronger attraction between the nucleus and the remaining electrons as the positive charge of the ion increases.
The Significance of High Ionization Energy:
The exceptionally high ionization energy of helium and other noble gases has profound implications:
-
Chemical Inertness: The high ionization energy contributes significantly to the chemical inertness of these elements. They are highly unreactive because it requires a substantial amount of energy to remove electrons and form chemical bonds.
-
Applications in Diverse Fields: Helium's unique properties, arising from its high ionization energy, find applications in cryogenics, welding, leak detection, and various scientific instruments.
-
Understanding Atomic Structure: The study of ionization energies provides invaluable insights into the structure of atoms, helping us to understand the forces that govern electron behavior and the stability of different electron configurations.
Conclusion: Helium's Reign Supreme
The element with the largest ionization energy is unequivocally Helium (He). Its exceptionally high first ionization energy stems from a confluence of factors: a small atomic radius, a high effective nuclear charge, minimal electron shielding, and the exceptional stability of its filled 1s² electron shell. This high ionization energy underpins helium’s chemical inertness and its broad applications across various scientific and technological domains. Understanding the factors influencing ionization energy provides a fundamental appreciation of atomic structure and the properties of the elements. The study of this essential property continues to contribute to advancements in chemistry, physics, and materials science.
Latest Posts
Latest Posts
-
How To Find A Collision Force
Mar 26, 2025
-
Is Diethyl Ether Soluble In Water
Mar 26, 2025
-
Are D Sugars More Abundant In Nature Than L Sugars
Mar 26, 2025
-
Energy Is Released When Bonds Are Broken
Mar 26, 2025
-
Express The Complex Number In Polar Form
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Element Has The Largest Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
