Energy Is Released When Bonds Are Broken
Muz Play
Mar 26, 2025 · 6 min read
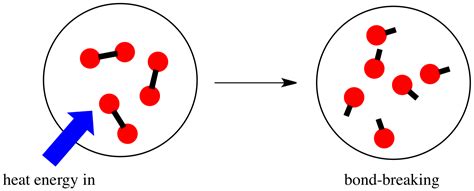
Table of Contents
Energy is Released When Bonds are Broken: A Deep Dive into Chemical Thermodynamics
The statement "energy is released when bonds are broken" is a common misconception in chemistry. While it's true that energy changes when bonds are broken, it's more accurate to say that energy is absorbed when bonds are broken, and energy is released when bonds are formed. This subtle but crucial difference is central to understanding chemical thermodynamics and reactions. This article will delve into the complexities of bond breaking and formation, exploring the energy changes involved, the different types of bonds, and the implications for various chemical processes.
Understanding Bond Energy
Chemical bonds represent the attractive forces holding atoms together in molecules and compounds. These bonds store potential energy. The amount of energy stored within a bond is known as bond energy or bond dissociation energy. It represents the energy required to break one mole of a particular type of bond in the gaseous phase, resulting in neutral atoms. It's crucial to remember that bond energies are average values, varying slightly depending on the molecular environment.
Exothermic vs. Endothermic Reactions
Chemical reactions involve the breaking of existing bonds and the formation of new ones. The overall energy change determines whether a reaction is exothermic or endothermic:
-
Exothermic Reactions: These reactions release energy to the surroundings. The energy released is greater than the energy absorbed. This means that the bonds formed in the products are stronger (and thus lower in energy) than the bonds broken in the reactants. The enthalpy change (ΔH) for an exothermic reaction is negative. A classic example is combustion.
-
Endothermic Reactions: These reactions absorb energy from the surroundings. The energy absorbed is greater than the energy released. This means that the bonds formed in the products are weaker (and thus higher in energy) than the bonds broken in the reactants. The enthalpy change (ΔH) for an endothermic reaction is positive. Photosynthesis is an example of an endothermic process.
The Process of Bond Breaking and Formation
Let's break down the energy changes at a molecular level:
Bond Breaking: An Energy-Requiring Process
Breaking a chemical bond always requires energy input. This energy overcomes the attractive forces holding the atoms together. The energy required varies depending on the type of bond and the atoms involved. For instance, breaking a strong covalent bond like the C=O double bond in carbon dioxide requires significantly more energy than breaking a weaker hydrogen bond in water. This energy input is often provided in the form of heat, light, or electricity.
Bond Formation: An Energy-Releasing Process
When atoms bond together to form new molecules, energy is released. This released energy is often in the form of heat, causing the surroundings to increase in temperature. The formation of stronger bonds releases more energy than the formation of weaker bonds. This energy release stabilizes the newly formed molecule.
The Net Energy Change: The Driving Force of Reactions
The overall energy change in a chemical reaction is the difference between the energy required to break bonds and the energy released when new bonds are formed. If more energy is released during bond formation than is absorbed during bond breaking, the reaction is exothermic. Conversely, if more energy is absorbed during bond breaking than is released during bond formation, the reaction is endothermic.
Types of Chemical Bonds and Their Energy
Different types of chemical bonds have varying bond energies:
Covalent Bonds
Covalent bonds involve the sharing of electrons between two atoms. The strength of a covalent bond depends on factors such as the electronegativity of the atoms involved and the bond order (single, double, or triple bond). Generally, double and triple bonds are stronger than single bonds.
- Single Bonds: These bonds are formed by sharing one pair of electrons. They are relatively weaker than multiple bonds.
- Double Bonds: Sharing two pairs of electrons results in a stronger, shorter bond.
- Triple Bonds: Sharing three pairs of electrons creates the strongest type of covalent bond.
Ionic Bonds
Ionic bonds are formed through the electrostatic attraction between oppositely charged ions (cations and anions). The strength of an ionic bond depends on the charge and size of the ions. Smaller, highly charged ions form stronger ionic bonds.
Hydrogen Bonds
Hydrogen bonds are relatively weak compared to covalent and ionic bonds. They occur between a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule. Hydrogen bonds play a crucial role in many biological systems.
Metallic Bonds
Metallic bonds are found in metals. They involve the delocalization of valence electrons among a lattice of metal atoms. The strength of metallic bonds varies depending on the metal.
Applications and Implications
Understanding the energy changes associated with bond breaking and formation is crucial in various fields:
Chemistry: Reaction Mechanisms and Kinetics
Knowing the bond energies of reactants and products helps predict the feasibility and kinetics of chemical reactions. The activation energy—the minimum energy required for a reaction to proceed—is directly related to the energy required to break specific bonds.
Biochemistry: Enzyme Catalysis and Metabolic Processes
Enzymes catalyze biological reactions by lowering the activation energy. They achieve this by facilitating bond breaking and formation in a way that requires less energy input. Understanding bond energies is essential for comprehending enzyme mechanisms and metabolic pathways.
Materials Science: Designing New Materials
The properties of materials are closely linked to the types of bonds present and their energies. Materials scientists utilize this knowledge to design materials with specific properties, such as strength, conductivity, or reactivity. For example, understanding bond strengths is crucial for creating strong polymers or highly conductive materials.
Environmental Science: Combustion and Pollution
Combustion reactions involve the breaking of bonds in fuel molecules and the formation of new bonds in products like carbon dioxide and water. Understanding the energy released during combustion is essential for developing more efficient and cleaner energy technologies. Additionally, understanding bond strengths helps in the design of strategies to mitigate pollution by efficiently breaking down harmful pollutants.
Conclusion: A Reframing of the Initial Statement
Returning to the initial statement, "energy is released when bonds are broken," we can now see its inaccuracy. While the statement is often used colloquially, it oversimplifies the complex thermodynamic processes involved in chemical reactions. Instead, we should focus on the nuanced interplay between bond breaking (an energy-requiring process) and bond formation (an energy-releasing process). The net energy change, determined by the difference between these two processes, dictates whether a reaction is exothermic or endothermic. A thorough understanding of bond energies, bond types, and their relationship to enthalpy changes is crucial for comprehending chemical reactions and their diverse applications across various scientific disciplines. The accurate understanding of these processes is fundamental for advancements in energy production, material science, medicine, and many other areas. The energy released is always a consequence of bond formation, not bond breakage. This subtle distinction underscores the importance of precise language in scientific discourse.
Latest Posts
Latest Posts
-
Does Oxidation Occur At The Anode
Mar 28, 2025
-
The Membrane Holds The Coils Of The Small Intestine
Mar 28, 2025
-
Organelles In Eukaryotic Cells Answer Key
Mar 28, 2025
-
Lewis Dot Diagram For Ionic Bonding Between Li And F
Mar 28, 2025
-
What Is The Relationship Between Avogadros Number And The Mole
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Energy Is Released When Bonds Are Broken . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
