Does Oxidation Occur At The Anode
Muz Play
Mar 28, 2025 · 5 min read
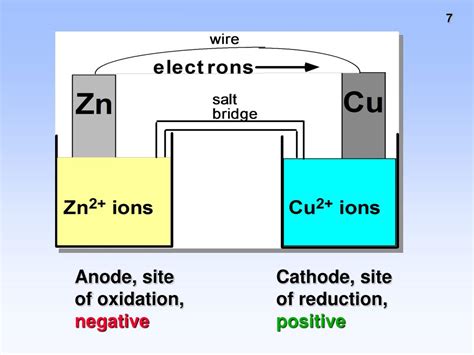
Table of Contents
Does Oxidation Occur at the Anode? A Deep Dive into Electrochemical Reactions
Understanding oxidation and reduction reactions, often abbreviated as redox reactions, is fundamental to comprehending electrochemistry. A common point of confusion for many students and even seasoned professionals involves the relationship between oxidation, reduction, the anode, and the cathode. This article will delve deep into this relationship, clarifying the statement: yes, oxidation occurs at the anode. We’ll explore the underlying principles, provide illustrative examples, and address common misconceptions.
Understanding Oxidation and Reduction
Before tackling the anode-oxidation relationship, let's solidify our understanding of oxidation and reduction themselves. These terms are best understood within the context of electron transfer:
-
Oxidation: This involves the loss of electrons by a species. When an atom, ion, or molecule loses electrons, its oxidation state increases. Remember the mnemonic device "OIL RIG": Oxidation Is Loss, Reduction Is Gain (of electrons).
-
Reduction: This involves the gain of electrons by a species. When an atom, ion, or molecule gains electrons, its oxidation state decreases.
These processes are always coupled; you cannot have oxidation without reduction, and vice versa. This is because electrons don't simply vanish; they are transferred from one species to another. This coupled nature is crucial for understanding electrochemical cells.
Electrochemical Cells: The Heart of Redox Reactions
Electrochemical cells are devices that harness the energy released during spontaneous redox reactions or use electrical energy to drive non-spontaneous redox reactions. They consist of two electrodes (anode and cathode) immersed in electrolytes (solutions containing ions). These cells can be categorized into two types:
-
Galvanic cells (voltaic cells): These cells generate electrical energy from spontaneous redox reactions. Think of batteries – they're galvanic cells.
-
Electrolytic cells: These cells use electrical energy to drive non-spontaneous redox reactions. Electroplating and the production of certain metals are examples of electrolytic processes.
The Anode: The Site of Oxidation
Now, we come to the crucial point. In all electrochemical cells, regardless of whether it's galvanic or electrolytic, oxidation always occurs at the anode. This is a fundamental principle of electrochemistry. The anode is defined as the electrode where oxidation takes place.
Why does oxidation occur at the anode?
The anode acts as a source of electrons. As oxidation involves the loss of electrons, the species undergoing oxidation releases electrons at the anode. These electrons then flow through the external circuit to the cathode. This electron flow constitutes the electric current.
The Cathode: The Site of Reduction
Conversely, reduction always occurs at the cathode. The cathode acts as a sink for electrons. The species undergoing reduction accepts electrons from the external circuit at the cathode. This completes the redox reaction.
Illustrative Examples: Galvanic Cells
Let’s consider a simple galvanic cell, the Daniell cell, composed of zinc and copper electrodes:
-
Anode (Zinc electrode): Zn(s) → Zn²⁺(aq) + 2e⁻ (Oxidation: Zinc loses electrons, becoming zinc ions)
-
Cathode (Copper electrode): Cu²⁺(aq) + 2e⁻ → Cu(s) (Reduction: Copper ions gain electrons, becoming copper metal)
In this cell, the zinc electrode is the anode because it undergoes oxidation. The electrons released by the zinc travel through the external circuit to the copper electrode (cathode), where they are accepted by copper ions to form copper metal. The overall reaction is: Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
Illustrative Examples: Electrolytic Cells
Let's examine an electrolytic cell, such as the electrolysis of molten sodium chloride:
-
Anode (Positive electrode): 2Cl⁻(l) → Cl₂(g) + 2e⁻ (Oxidation: Chloride ions lose electrons, forming chlorine gas)
-
Cathode (Negative electrode): Na⁺(l) + e⁻ → Na(l) (Reduction: Sodium ions gain electrons, forming sodium metal)
Here, the chloride ions at the anode lose electrons to become chlorine gas, demonstrating oxidation at the anode. The sodium ions at the cathode gain electrons to form sodium metal, demonstrating reduction at the cathode. The overall reaction is: 2NaCl(l) → 2Na(l) + Cl₂(g)
Common Misconceptions
A frequent source of confusion arises from the terminology:
-
Positive and Negative Electrodes: While in galvanic cells, the anode is often negatively charged and the cathode positively charged, this is not always true and should not be used as a defining characteristic. In electrolytic cells, the anode is positive and the cathode is negative. The charge of the electrode depends on the direction of electron flow which, in turn, depends on the type of cell. Focus on the processes (oxidation and reduction) occurring at each electrode, not just their charge.
-
Remembering Anode and Cathode: Many students struggle to remember which electrode is the anode and which is the cathode. A simple mnemonic is "An Ox, Red Cat": Anode – Oxidation, Reduction – Cathode.
Beyond the Basics: Factors Influencing Electrode Potentials
The potential difference between the anode and cathode, also known as the cell potential, is crucial for determining the spontaneity of a redox reaction. Several factors influence these electrode potentials:
-
Standard Reduction Potentials: These potentials are measured under standard conditions (298K, 1 atm pressure, 1M concentration). They provide a relative measure of the tendency of a species to be reduced.
-
Concentration Effects: The Nernst equation describes how changes in the concentration of reactants and products affect the cell potential.
-
Temperature: Temperature influences the rate of electron transfer and the overall cell potential.
Conclusion: Oxidation's Inseparable Link to the Anode
In conclusion, oxidation invariably occurs at the anode in all electrochemical cells. This is not merely a convention; it's a fundamental consequence of how electrons flow during redox reactions. Understanding this relationship is crucial for mastering electrochemistry, designing electrochemical cells, and interpreting the processes occurring within them. By focusing on the electron transfer processes – oxidation at the anode and reduction at the cathode – and avoiding reliance on electrode charge as the primary defining feature, one can build a strong and reliable understanding of this fundamental concept. The examples provided, coupled with clarifying the common misconceptions, ensure a comprehensive understanding of the relationship between oxidation and the anode in the realm of electrochemistry.
Latest Posts
Latest Posts
-
What Happens To An Animal Cell In A Isotonic Solution
Mar 31, 2025
-
Atom That Has Gained Or Lost Electrons
Mar 31, 2025
-
Cuantas Onzas Tiene Un Cuarto De Galon
Mar 31, 2025
-
Work Done By A Varying Force
Mar 31, 2025
-
The Rna Components Of Ribosomes Are Synthesized In The
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Does Oxidation Occur At The Anode . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
