What Happens To Pressure When Volume Increases
Muz Play
Mar 24, 2025 · 6 min read
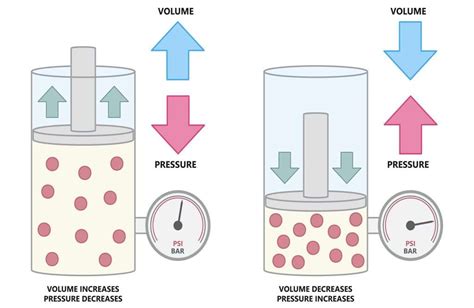
Table of Contents
What Happens to Pressure When Volume Increases? An In-Depth Exploration of Boyle's Law and Beyond
Understanding the relationship between pressure and volume is fundamental to comprehending many physical phenomena, from the workings of our lungs to the behavior of gases in industrial processes. This article delves deep into the principle governing this relationship – Boyle's Law – and explores its implications, limitations, and extensions to more complex scenarios.
Boyle's Law: The Foundation of Pressure-Volume Relationships
At the heart of understanding what happens to pressure when volume increases lies Boyle's Law, a cornerstone of gas laws. This law states that for a fixed amount of gas at a constant temperature, the pressure (P) and volume (V) are inversely proportional. Mathematically, this is represented as:
P₁V₁ = P₂V₂
where:
- P₁ and V₁ represent the initial pressure and volume.
- P₂ and V₂ represent the final pressure and volume.
This equation beautifully encapsulates the core concept: if the volume of a gas increases, its pressure decreases, and vice versa, provided the temperature and amount of gas remain unchanged.
Visualizing Boyle's Law
Imagine a sealed container filled with gas. If you increase the container's volume (perhaps by expanding a piston), the gas molecules have more space to move around. This leads to fewer collisions between the gas molecules and the container walls per unit time. Since pressure is essentially the force exerted by these collisions, a decrease in collision frequency results in a decrease in pressure. Conversely, compressing the gas (reducing the volume) forces the molecules closer together, increasing the collision frequency and thus the pressure.
Real-World Examples of Boyle's Law
Boyle's Law manifests itself in countless everyday situations:
- Breathing: Our lungs operate based on this principle. When we inhale, the diaphragm contracts, increasing the volume of our lungs and decreasing the air pressure inside. This pressure difference causes air to rush into our lungs. Exhalation is the reverse process.
- Inflatable Balloons: Inflating a balloon involves increasing the volume of air inside, thereby decreasing its pressure relative to the surrounding atmosphere. Deflating the balloon reverses this process.
- Diving: As divers descend, the pressure of the surrounding water increases, causing the volume of air in their lungs and equipment to decrease. This highlights the importance of controlled ascents to prevent decompression sickness.
- Syringes: Pulling the plunger of a syringe increases the volume of the barrel, reducing the pressure inside and drawing liquid into it. Pushing the plunger reverses this effect.
- Pneumatic Systems: Many industrial and automotive systems utilize compressed air, leveraging Boyle's Law to generate power or control movements. The pressure in these systems is directly related to the volume of compressed air.
Beyond Boyle's Law: Considering Temperature and the Amount of Gas
While Boyle's Law provides a crucial understanding of the pressure-volume relationship, it's important to remember its limitations. It only holds true under the ideal gas conditions, assuming the following:
- Constant Temperature: Temperature fluctuations affect the kinetic energy of gas molecules, influencing both pressure and volume.
- Fixed Amount of Gas: Adding or removing gas changes the number of molecules, thereby affecting pressure and volume.
- Ideal Gas Behavior: The law assumes gas molecules have negligible size and exert no intermolecular forces. This is an idealization that works well for many gases at moderate pressures and temperatures but breaks down at high pressures or low temperatures where intermolecular forces become significant.
To account for temperature and the amount of gas, we need the Ideal Gas Law, a more comprehensive equation:
PV = nRT
Where:
- P = Pressure
- V = Volume
- n = Number of moles of gas
- R = Ideal gas constant
- T = Absolute temperature (in Kelvin)
The Ideal Gas Law provides a more accurate description of gas behavior under a wider range of conditions. It incorporates Boyle's Law as a special case where n and T are held constant.
The Effects of Temperature on Pressure-Volume Relationships
Temperature plays a significant role in modifying the pressure-volume relationship. Consider the following scenarios:
- Constant Volume, Increasing Temperature: If we heat a gas at a constant volume, the kinetic energy of the molecules increases, causing them to collide with the container walls more frequently and with greater force. This results in an increase in pressure.
- Constant Pressure, Increasing Temperature: If we heat a gas at a constant pressure, the increased kinetic energy leads to an expansion in volume as the gas molecules move farther apart.
- Isothermal Processes: Processes conducted at constant temperature follow Boyle's Law directly. The pressure and volume are inversely proportional.
- Isobaric Processes: Processes conducted at constant pressure show a direct relationship between volume and temperature (Charles's Law).
- Isochoric Processes: Processes conducted at constant volume show a direct relationship between pressure and temperature (Gay-Lussac's Law).
The Impact of the Amount of Gas
Adding more gas to a container at a constant temperature and volume will increase the pressure. This is because there are more molecules colliding with the container walls per unit time, leading to a higher force and thus higher pressure. Conversely, removing gas will decrease the pressure.
Real Gases vs. Ideal Gases: Departures from Boyle's Law
Boyle's Law and the Ideal Gas Law provide excellent approximations for the behavior of many gases under ordinary conditions. However, at high pressures and low temperatures, real gases deviate from ideal behavior. This is because:
- Finite Molecular Size: Real gas molecules occupy a finite volume, reducing the available space for movement compared to the assumption in ideal gas laws.
- Intermolecular Forces: Attractive forces between gas molecules, like van der Waals forces, can affect their motion and reduce the frequency of collisions with the container walls, leading to lower pressure than predicted by the ideal gas law.
These deviations are usually addressed by using equations of state, such as the van der Waals equation, which incorporate correction factors to account for the finite size and intermolecular interactions of real gas molecules.
Applications in Various Fields
The principles governing the relationship between pressure and volume have far-reaching applications across numerous fields:
- Meteorology: Understanding pressure-volume changes in the atmosphere is crucial for weather forecasting and climate modeling.
- Engineering: Designing and operating systems involving compressed gases, such as pneumatic systems, requires a thorough understanding of Boyle's Law and the Ideal Gas Law.
- Medicine: Respiratory therapy and anesthesia rely on understanding the pressure-volume relationships in the lungs and the body's response to changes in gas pressure.
- Chemistry: Many chemical reactions involve gases, and understanding the pressure-volume relationships is essential for controlling reaction rates and yields.
Conclusion: A Dynamic Relationship
The relationship between pressure and volume is a fundamental aspect of gas behavior. While Boyle's Law provides a simple yet powerful initial understanding, the Ideal Gas Law offers a more comprehensive description, incorporating temperature and the amount of gas. Understanding these principles is critical for comprehending numerous phenomena in everyday life and various scientific and engineering applications. Furthermore, acknowledging the limitations of ideal gas laws and considering the behavior of real gases provides a more complete picture of this dynamic relationship and its implications. By appreciating the complexities and nuances of pressure-volume interactions, we can better predict and control gas behavior in diverse contexts.
Latest Posts
Latest Posts
-
Chemical Energy For Respiration Is Stored In The Bonds Of
Mar 26, 2025
-
He Is Mehe Is My Insphe Is My Father
Mar 26, 2025
-
An Environment Where Oxygen Is Absent Is Termed
Mar 26, 2025
-
What 3 Subatomic Particles Make Up An Atom
Mar 26, 2025
-
Proteins Are Made Up Of Monomers Called
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Happens To Pressure When Volume Increases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
