What Has A Definite Shape And Volume
Muz Play
Mar 22, 2025 · 5 min read
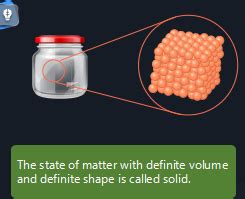
Table of Contents
What Has a Definite Shape and Volume? Exploring the World of Solids
Understanding the properties of matter is fundamental to grasping the physical world around us. One crucial distinction lies in the classification of matter based on its shape and volume. This article delves deep into the characteristics of matter that possesses a definite shape and volume, focusing primarily on solids. We'll explore the reasons behind their rigidity, the various types of solids, and the fascinating interplay of forces that dictate their behavior.
The Defining Characteristics: Definite Shape and Volume
The simple answer to the question "What has a definite shape and volume?" is a solid. Unlike liquids and gases, solids exhibit two key properties:
-
Definite Shape: A solid retains its shape regardless of its container. It doesn't conform to the shape of its surroundings. A block of wood remains a block of wood whether it's placed on a table or in a box.
-
Definite Volume: A solid occupies a fixed amount of space. Its volume remains constant under normal conditions of temperature and pressure. You can't easily compress a solid to significantly reduce its volume.
The Microscopic Explanation: Intermolecular Forces
The reason solids possess these properties lies at the microscopic level. The atoms or molecules within a solid are held together by strong intermolecular forces. These forces are significantly stronger than those in liquids or gases. This strong attraction results in a highly ordered arrangement of particles, often forming a lattice structure. The particles are tightly packed and vibrate in fixed positions, preventing the solid from easily changing its shape or volume.
Types of Intermolecular Forces:
The strength of the intermolecular forces significantly influences the properties of the solid. Various types exist, including:
-
Ionic Bonds: These are strong electrostatic forces between oppositely charged ions. They are found in ionic compounds like sodium chloride (table salt). Ionic solids are typically hard and brittle.
-
Covalent Bonds: These involve the sharing of electron pairs between atoms. They are strong and are responsible for the formation of many molecular solids, like diamond or quartz. Covalent solids can exhibit a wide range of properties depending on the arrangement of the atoms.
-
Metallic Bonds: These are found in metals. The valence electrons are delocalized, forming a "sea" of electrons that surrounds the positively charged metal ions. This gives metals their characteristic properties like high electrical and thermal conductivity, malleability, and ductility.
-
Van der Waals Forces: These are weaker forces that arise from temporary fluctuations in electron distribution. They are present in many molecular solids and play a role in determining their melting points and other physical properties. These forces are weaker than ionic, covalent, or metallic bonds.
Exploring the Diverse World of Solids: Types of Solids
Solids can be further categorized based on their internal structure and bonding:
1. Crystalline Solids: Order and Precision
Crystalline solids are characterized by a highly ordered, repeating three-dimensional arrangement of atoms, ions, or molecules. This arrangement, known as a crystal lattice, gives rise to their distinct properties. Examples include:
-
Ionic Crystals: Sodium chloride (NaCl), potassium bromide (KBr). These are generally hard, brittle, and have high melting points.
-
Covalent Crystals: Diamond (C), quartz (SiO₂). These are extremely hard and have very high melting points.
-
Metallic Crystals: Iron (Fe), copper (Cu), gold (Au). These are often malleable, ductile, and good conductors of heat and electricity.
-
Molecular Crystals: Ice (H₂O), sugar (C₁₂H₂₂O₁₁). These have relatively low melting points and are often soft.
The specific arrangement of atoms in a crystal lattice dictates many of its physical properties, including its hardness, melting point, and cleavage planes.
2. Amorphous Solids: Disorder and Irregularity
In contrast to crystalline solids, amorphous solids lack a well-defined, long-range order in their atomic or molecular arrangement. Their structure is more disordered and irregular. Examples include:
-
Glass: A supercooled liquid, glass lacks the crystalline structure of a solid but maintains a rigid shape.
-
Plastics: Many polymers form amorphous solids with tangled chains of molecules.
-
Rubber: Another example of an amorphous solid with a flexible, disordered structure.
Amorphous solids generally have lower melting points than crystalline solids and don't exhibit sharp melting points. They often soften gradually over a temperature range.
Factors Affecting the Properties of Solids
Several factors contribute to the overall properties of a solid:
-
Strength of Intermolecular Forces: Stronger forces lead to harder, higher-melting-point solids.
-
Type of Bonding: Ionic, covalent, metallic, and Van der Waals bonds all contribute differently to the solid's properties.
-
Crystal Structure: The arrangement of atoms or molecules in the crystal lattice significantly influences hardness, cleavage, and other physical properties.
-
Impurities: The presence of impurities can alter the properties of a solid, sometimes drastically. Alloying metals, for instance, can significantly increase their strength and hardness.
-
Temperature and Pressure: Changes in temperature and pressure can affect the arrangement of atoms and molecules, altering the properties of the solid. For example, increasing pressure can make a solid denser.
The Significance of Understanding Solids
The study of solids is crucial across a wide range of scientific and technological disciplines. Understanding their properties is vital in:
-
Materials Science: Developing new materials with desired properties, such as strength, conductivity, and durability.
-
Engineering: Designing structures and components that can withstand stress and strain.
-
Chemistry: Understanding chemical reactions and processes involving solids.
-
Geology: Studying the formation and properties of rocks and minerals.
-
Physics: Investigating the fundamental forces and interactions within solids.
Conclusion: A Solid Foundation
Solids, with their definite shape and volume, represent a fundamental state of matter with diverse properties and applications. Their behavior is governed by the intricate interplay of intermolecular forces, crystal structure, and external factors. By understanding the microscopic and macroscopic characteristics of solids, we can unlock their potential and harness their remarkable properties for various technological advancements and scientific breakthroughs. The world of solids is a rich and fascinating field of study, continuously revealing new insights and possibilities.
Latest Posts
Latest Posts
-
Is Work The Integral Of Force
Mar 23, 2025
-
Has A Definite Shape And Volume
Mar 23, 2025
-
How Is The Circulatory System Similar To A Road And Highway System
Mar 23, 2025
-
Diels Alder Reaction With Maleic Anhydride
Mar 23, 2025
-
Center Of Mass Of A Hemisphere
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Has A Definite Shape And Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
