What Has No Definite Shape Or Volume
Muz Play
Apr 04, 2025 · 7 min read
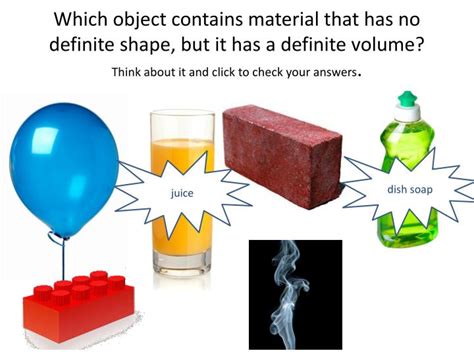
Table of Contents
What Has No Definite Shape or Volume? Exploring the World of Gases
The question, "What has no definite shape or volume?" leads us directly to the fascinating world of gases. Unlike solids with their rigid structures and liquids with their flowing but defined volumes, gases are unique in their ability to conform completely to the shape and volume of their container. This lack of a definite shape and volume stems from the fundamental properties of gas molecules and their interactions. Understanding this characteristic opens a door to a wealth of scientific concepts, from atmospheric pressure to the behavior of ideal gases.
Understanding the Nature of Gases
Gases are one of the four fundamental states of matter, alongside solids, liquids, and plasmas. Their defining characteristic is the weak intermolecular forces between their constituent particles (atoms or molecules). This means the particles are far apart compared to those in solids or liquids, and they move freely and randomly. This constant, chaotic motion is what gives gases their distinctive properties.
The Kinetic Molecular Theory of Gases
The behavior of gases is best explained by the kinetic molecular theory (KMT). This theory postulates that:
- Gases are composed of tiny particles (atoms or molecules) that are in constant, random motion. These particles are in continuous, chaotic movement, colliding with each other and the walls of their container.
- The volume of these particles is negligible compared to the volume of the container they occupy. The vast spaces between gas particles are a key factor in their lack of definite shape and volume.
- The attractive and repulsive forces between gas particles are negligible. While some attractive forces exist, especially at lower temperatures and higher pressures, they are generally weak compared to the kinetic energy of the particles.
- Gas particle collisions are perfectly elastic. This means that no kinetic energy is lost during collisions; energy is simply transferred between particles.
- The average kinetic energy of the gas particles is directly proportional to the absolute temperature of the gas. This means that as temperature increases, so does the speed and energy of the gas particles.
These postulates explain why gases expand to fill their containers. The particles are not bound to fixed positions and move freely, constantly bouncing off the container walls, effectively distributing themselves evenly throughout the available space. The absence of significant intermolecular forces allows them to spread out indefinitely.
Factors Affecting Gas Behavior
Several factors influence the behavior of gases, impacting their pressure, volume, temperature, and the number of moles present. These relationships are beautifully described by gas laws.
Pressure (P)
Pressure is the force exerted by gas particles per unit area on the walls of their container. It's directly related to the frequency and force of these collisions. Higher temperature means more energetic collisions and thus higher pressure. A larger number of gas particles in the same volume will also lead to higher pressure as there are more collisions.
Volume (V)
Volume is the space occupied by the gas. As mentioned, gases expand to fill their containers, so the volume of a gas is simply the volume of its container. Increasing the volume while keeping the temperature and number of particles constant will result in lower pressure as the particles have more space to move around in and collide less frequently.
Temperature (T)
Temperature is a measure of the average kinetic energy of the gas particles. Higher temperature means higher kinetic energy, leading to more frequent and forceful collisions, thus increasing the pressure. Conversely, lowering the temperature reduces the kinetic energy and pressure. Temperature is always measured in Kelvin (K) in gas law calculations.
Number of Moles (n)
The number of moles (n) represents the amount of gas present. Increasing the number of gas particles at constant volume and temperature will lead to an increase in pressure due to the increased number of collisions.
The Ideal Gas Law: A Powerful Relationship
The relationships between pressure, volume, temperature, and number of moles are elegantly summarized by the ideal gas law:
PV = nRT
where:
- P = pressure
- V = volume
- n = number of moles
- R = the ideal gas constant (a proportionality constant)
- T = temperature in Kelvin
This equation describes the behavior of an ideal gas, a theoretical gas whose particles have negligible volume and no intermolecular forces. While real gases deviate from ideal behavior, especially at high pressures and low temperatures, the ideal gas law provides a good approximation for many situations.
Deviations from Ideal Behavior: Real Gases
Real gases, unlike ideal gases, do experience intermolecular forces and their particles do have a finite volume. These factors cause deviations from the ideal gas law, particularly at high pressures and low temperatures.
High Pressure
At high pressures, the gas particles are compressed closer together. The volume occupied by the particles themselves becomes significant compared to the total volume of the container, leading to a smaller available volume for movement. Furthermore, the intermolecular forces become more pronounced as particles are in closer proximity. These factors cause the pressure to be higher than predicted by the ideal gas law.
Low Temperature
At low temperatures, the kinetic energy of the gas particles is reduced. The attractive intermolecular forces become more significant compared to the kinetic energy, causing the particles to stick together slightly. This reduces the pressure compared to the ideal gas law prediction.
Applications of Gas Behavior
The understanding of gas behavior has numerous practical applications across various fields:
Meteorology: Weather Prediction
The behavior of gases in the Earth's atmosphere, including pressure, temperature, and humidity, is crucial for weather forecasting. Meteorological models rely heavily on gas laws to predict weather patterns.
Chemistry: Gas Reactions
Chemical reactions often involve gases as reactants or products. Gas laws are essential in stoichiometry calculations and determining reaction yields.
Engineering: Design of Engines and Other Systems
Gas laws are essential in designing internal combustion engines, gas turbines, and other systems involving compressed gases. Understanding the relationship between pressure, volume, and temperature is crucial for optimizing efficiency and safety.
Medicine: Respiratory Therapy
The principles of gas behavior are applied in respiratory therapy, particularly in understanding the mechanics of breathing and the delivery of oxygen and other gases to patients.
Environmental Science: Atmospheric Pollution
Understanding the behavior of gases in the atmosphere is crucial for studying and mitigating atmospheric pollution. Gas laws are used to model the dispersion and transport of pollutants.
Beyond the Basics: Advanced Concepts
The study of gases extends beyond the simple ideal gas law. More advanced concepts include:
- Partial Pressures: The pressure exerted by an individual gas in a mixture of gases. Dalton's law of partial pressures states that the total pressure of a gas mixture is the sum of the partial pressures of the individual gases.
- Effusion and Diffusion: Effusion refers to the escape of gas particles through a small hole, while diffusion refers to the mixing of gases. Graham's law describes the relationship between the rates of effusion and diffusion and the molar masses of the gases involved.
- Real Gas Equations: Equations like the van der Waals equation attempt to account for the non-ideal behavior of real gases, providing a more accurate description of their behavior at high pressures and low temperatures.
Conclusion
The question of what has no definite shape or volume leads us to a deep understanding of the nature of gases. Their unique properties, stemming from the weak intermolecular forces and the constant motion of their particles, are elegantly explained by the kinetic molecular theory and the ideal gas law. While the ideal gas law provides a useful approximation, understanding the deviations exhibited by real gases is critical for numerous practical applications in diverse fields. The study of gases remains a vibrant area of research, with ongoing investigations into complex gas mixtures and their behavior under extreme conditions, expanding our understanding of this fundamental state of matter.
Latest Posts
Latest Posts
-
Are Lysosomes Only In Animal Cells
Apr 05, 2025
-
Which Describes An Object In Projectile Motion
Apr 05, 2025
-
What Is The Rate Determining Step Of A Reaction
Apr 05, 2025
-
What Is The Function Of The Parental Dna In Replication
Apr 05, 2025
-
Quantum Mechanical Model Vs Bohr Model
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about What Has No Definite Shape Or Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
