What Influences The Rate Of Diffusion
Muz Play
Mar 21, 2025 · 6 min read
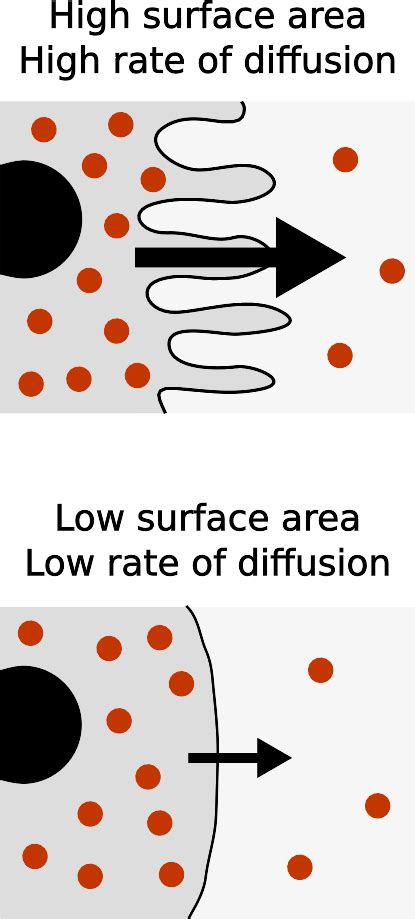
Table of Contents
What Influences the Rate of Diffusion? A Comprehensive Guide
Diffusion, the net movement of particles from a region of higher concentration to a region of lower concentration, is a fundamental process in many areas of science and everyday life. Understanding the factors that influence the rate of diffusion is crucial in fields ranging from biology (cellular transport) and chemistry (reaction rates) to engineering (material science) and environmental science (pollution dispersal). This comprehensive guide delves into the key factors governing diffusion rates, providing a detailed explanation of each and illustrating their impact with relevant examples.
1. Concentration Gradient: The Driving Force of Diffusion
The concentration gradient, the difference in concentration between two regions, is the primary driving force behind diffusion. A steeper concentration gradient (a larger difference in concentration) results in a faster rate of diffusion. This is because there's a greater driving force pushing particles from the high-concentration area to the low-concentration area. Imagine dropping a dye tablet into a glass of water. The dye initially concentrates around the tablet, creating a steep concentration gradient. The dye particles rapidly spread outwards, driven by this gradient. As the dye disperses, the concentration gradient lessens, and the rate of diffusion slows down.
Understanding Fick's First Law
Fick's First Law of diffusion mathematically describes this relationship:
J = -D (dC/dx)
Where:
- J represents the diffusion flux (amount of substance diffusing per unit area per unit time).
- D is the diffusion coefficient (a measure of how easily a substance diffuses through a medium).
- dC/dx represents the concentration gradient (the change in concentration over distance).
The negative sign indicates that diffusion occurs in the direction of decreasing concentration. This equation clearly highlights the direct proportionality between the diffusion flux and the concentration gradient – a larger gradient leads to a higher flux (faster diffusion).
2. Temperature: Kinetic Energy and Molecular Movement
Temperature significantly influences diffusion rates. Higher temperatures equate to increased kinetic energy of the particles. With greater kinetic energy, particles move faster and collide more frequently, leading to a faster rate of diffusion. This is because the increased energy overcomes the intermolecular forces holding the particles together, enabling them to spread more readily.
Consider the example of sugar dissolving in hot versus cold water. Sugar dissolves much faster in hot water because the increased kinetic energy of water molecules allows them to interact more effectively with the sugar crystals, breaking them down and dispersing the sugar molecules throughout the solution more quickly.
3. Mass of the Diffusing Particles: Size Matters
The mass of the diffusing particles directly affects the diffusion rate. Smaller particles diffuse faster than larger particles because they possess greater kinetic energy at the same temperature and encounter less resistance as they move through the medium. Larger, heavier molecules move more slowly and encounter more frequent collisions, hindering their movement.
This principle is evident in comparing the diffusion of gases versus liquids. Gas molecules, being significantly smaller and less densely packed, generally diffuse much faster than liquid molecules. Similarly, within a liquid, smaller solute molecules will diffuse faster than larger ones.
4. Medium of Diffusion: The Role of Viscosity and Permeability
The medium through which diffusion occurs plays a crucial role. The viscosity (thickness) of the medium impacts diffusion rates. Less viscous media allow for faster diffusion because particles encounter less resistance as they move. High viscosity, on the other hand, impedes particle movement, slowing down diffusion.
For example, diffusion is faster in water (low viscosity) than in honey (high viscosity). The permeability of the medium is also important. A more permeable medium allows particles to pass through more easily, increasing the rate of diffusion. A less permeable medium acts as a barrier, hindering diffusion. Think of the difference between diffusion across a cell membrane (relatively permeable) versus diffusion through a thick, impermeable wall.
5. Surface Area: More Area, Faster Diffusion
The surface area available for diffusion significantly influences the rate. A larger surface area provides more pathways for particles to move, resulting in faster diffusion. A smaller surface area restricts the number of pathways, slowing down the process.
Imagine comparing the diffusion of a solute into a large container versus a small container. The larger container, with its larger surface area, will allow the solute to diffuse more rapidly. This principle is exploited in many biological systems, such as the highly folded structure of the inner mitochondrial membrane, which maximizes the surface area for efficient respiration.
6. Distance of Diffusion: The Impact of Path Length
The distance over which diffusion occurs also affects the rate. Diffusion is inherently a slow process, and the rate decreases as the distance increases. Particles have to travel further, increasing the time taken for significant concentration changes. This is why efficient transport mechanisms are crucial in organisms – relying solely on diffusion over long distances would be far too slow for many biological processes.
For instance, the transport of oxygen from the lungs to the tissues in larger organisms relies on the circulatory system, as diffusion alone would be insufficient to cover the necessary distance quickly enough.
7. Pressure: Influence on Gases
In the case of gases, pressure plays a significant role. Higher pressure means a higher concentration of gas molecules in a given volume. This increased concentration leads to a steeper concentration gradient, consequently accelerating the rate of diffusion. Conversely, lower pressure results in slower diffusion.
This is why gases diffuse more rapidly at higher altitudes, where the atmospheric pressure is lower. Conversely, increased pressure, as seen in deep-sea environments, can compress gases, affecting their diffusion rates.
8. Presence of Other Substances: Competitive and Inhibitory Effects
The presence of other substances in the diffusing medium can influence the rate of diffusion. Other molecules can either compete for space and pathways, slowing diffusion down (competitive inhibition), or they can interact directly with the diffusing particles, hindering their movement (inhibitory effects). This becomes crucial in complex systems like cells, where many different molecules are simultaneously diffusing. The interactions between these molecules can significantly alter overall diffusion rates.
Conclusion: A Complex Interplay of Factors
The rate of diffusion isn't governed by a single factor but by a complex interplay of several interconnected variables. Understanding these influences is vital for comprehending various phenomena in nature and for designing and optimizing processes in various fields of study. From the design of drug delivery systems to understanding the transport of nutrients in plants, the principles of diffusion are fundamental to numerous applications. By carefully controlling the relevant parameters, we can manipulate and optimize diffusion rates for desired outcomes. Future research will continue to refine our understanding of this critical process and its implications across scientific disciplines.
Latest Posts
Latest Posts
-
How Are Pressure And Temp Related
Mar 21, 2025
-
Is Burning Gasoline A Chemical Change
Mar 21, 2025
-
Which Is The Central Element For All Living Things
Mar 21, 2025
-
Acid And Base Extraction Lab Report
Mar 21, 2025
-
Where Are Prolines Found On An Alpha Helix
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Influences The Rate Of Diffusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
