What Is A Characteristic Of A Base
Muz Play
Apr 07, 2025 · 6 min read
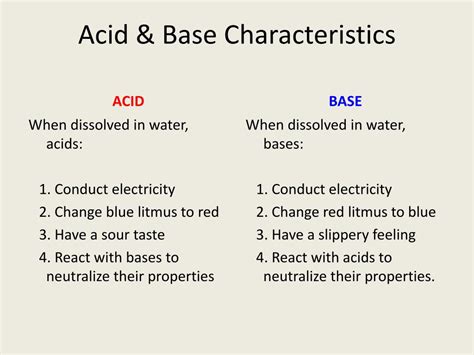
Table of Contents
What is a Characteristic of a Base? Understanding the Properties of Bases
Bases are fundamental chemical compounds with distinct properties that set them apart from acids. Understanding these characteristics is crucial in various fields, from chemistry and biology to environmental science and everyday life. This comprehensive guide dives deep into the defining characteristics of bases, exploring their chemical behavior, practical applications, and safety considerations.
Defining Bases: More Than Just the Opposite of Acids
While often presented as simply the opposite of acids, bases possess unique characteristics that define their nature and behavior. The most common definition centers around their ability to:
-
Accept protons (H⁺): According to the Brønsted-Lowry theory, a base is a substance that accepts a proton (a hydrogen ion) from an acid. This proton acceptance is the core of many base reactions. For example, ammonia (NH₃) acts as a base by accepting a proton from water (H₂O), forming ammonium (NH₄⁺) and hydroxide (OH⁻) ions.
-
Donate hydroxide ions (OH⁻): Arrhenius theory defines a base as a substance that increases the concentration of hydroxide ions (OH⁻) in an aqueous solution. This hydroxide ion release is responsible for the characteristic properties of many common bases, like sodium hydroxide (NaOH) or potassium hydroxide (KOH). These strong bases readily dissociate in water, releasing a significant amount of OH⁻ ions.
-
Have a pH greater than 7: The pH scale measures the acidity or basicity of a solution. A pH of 7 is considered neutral. Bases have a pH greater than 7, with stronger bases exhibiting higher pH values. The higher the pH, the more basic the solution.
Key Characteristics of Bases: A Detailed Exploration
Beyond the fundamental definitions, several key characteristics help identify and understand bases:
1. Taste and Feel: Bitter and Slippery
Many bases exhibit a bitter taste and a slippery or soapy feel when in contact with skin. This is due to the reaction of the base with the oils and proteins on the skin, leading to a change in texture. Caution: Never taste or directly touch unknown chemicals, as many bases can be corrosive and harmful.
2. Reaction with Acids: Neutralization Reactions
A hallmark of bases is their reaction with acids. This reaction, known as neutralization, produces salt and water. The heat released during this reaction is often noticeable, indicating an exothermic process. The general equation for a neutralization reaction is:
Acid + Base → Salt + Water
For example:
HCl (Hydrochloric acid) + NaOH (Sodium hydroxide) → NaCl (Sodium chloride) + H₂O (Water)
3. Effect on Indicators: Changing Colors
Bases change the color of acid-base indicators. These indicators are substances that change color depending on the pH of the solution. Common examples include litmus paper, phenolphthalein, and methyl orange. Bases typically turn litmus paper blue, phenolphthalein pink, and methyl orange yellow. This color change is a convenient way to identify the presence of a base.
4. Electrical Conductivity: Conducting Solutions
Many bases, particularly strong bases, are good conductors of electricity when dissolved in water. This is because they dissociate into ions (cations and anions), which carry an electric charge and facilitate the flow of current. The higher the concentration of ions, the better the conductivity. Weak bases, however, conduct electricity less effectively due to their lower degree of dissociation.
5. Reactivity with Metals: Specific Reactions
Some bases react with certain metals, particularly amphoteric metals like aluminum and zinc, producing hydrogen gas. This reaction demonstrates the base's ability to abstract protons from the metal, leading to the formation of hydrogen gas and a metal salt. This reaction is often slow and may require heat to initiate.
Example: 2Al + 2NaOH + 6H₂O → 2Na[Al(OH)₄] + 3H₂
Types of Bases: A Spectrum of Strength and Properties
Bases are categorized into different types based on their strength and properties:
1. Strong Bases: Complete Dissociation
Strong bases are those that completely dissociate in water, releasing a high concentration of hydroxide ions. Examples include:
- Sodium hydroxide (NaOH): Commonly known as caustic soda or lye, it's a widely used base in various industries.
- Potassium hydroxide (KOH): Similar to NaOH in its properties, it's another strong base with various applications.
- Calcium hydroxide (Ca(OH)₂): Also known as slaked lime, it's less soluble than NaOH or KOH but still considered a strong base.
2. Weak Bases: Partial Dissociation
Weak bases only partially dissociate in water, producing a lower concentration of hydroxide ions. Examples include:
- Ammonia (NH₃): A common household cleaning agent, it reacts with water to form a small amount of ammonium and hydroxide ions.
- Sodium carbonate (Na₂CO₃): Also known as washing soda, it's a weaker base compared to NaOH or KOH.
- Many organic amines: These nitrogen-containing compounds act as weak bases due to the presence of a lone pair of electrons on the nitrogen atom.
3. Superbases: Extremely Strong Bases
Superbases are exceptionally strong bases that can deprotonate even very weak acids. They are often used in specialized chemical reactions requiring extremely high basicity.
Applications of Bases: A Wide Range of Uses
Bases find applications across a vast range of fields:
1. Industrial Applications: Manufacturing and Production
Bases are extensively used in various industrial processes, including:
- Chemical manufacturing: Many industrial chemicals are synthesized using bases as catalysts or reactants.
- Soap and detergent production: Saponification, a process involving the reaction of fats with a strong base, is used to produce soaps.
- Paper manufacturing: Bases are used in the pulping process to break down wood fibers.
- Wastewater treatment: Bases help neutralize acidic wastewater, preventing environmental damage.
2. Everyday Applications: Household and Personal Care
Many common household items and personal care products contain bases:
- Cleaning agents: Many household cleaners contain bases to dissolve grease and grime.
- Soaps and detergents: These are commonly used for cleaning purposes and rely on the properties of bases.
- Antacids: Some antacids contain bases to neutralize stomach acid.
3. Biological Applications: Maintaining pH Balance
Bases play a critical role in biological systems:
- Maintaining blood pH: The body uses buffers to maintain the proper blood pH, a process involving weak bases and acids.
- Enzyme activity: The activity of many enzymes depends on the pH of the environment, which is often regulated by bases.
Safety Considerations: Handling Bases with Care
Many bases are corrosive and can cause serious harm if not handled properly. Always follow these safety precautions:
- Wear appropriate personal protective equipment (PPE): This includes gloves, eye protection, and lab coats to minimize contact with skin and eyes.
- Work in a well-ventilated area: Some bases release harmful fumes.
- Avoid contact with skin and eyes: If contact occurs, immediately rinse the affected area with plenty of water.
- Store bases safely: Keep bases away from acids and incompatible materials.
- Follow proper disposal procedures: Dispose of bases according to local regulations.
Conclusion: Understanding the Power of Bases
Bases are essential chemical compounds with a wide range of applications and properties. Understanding their characteristics, from their proton-accepting ability to their reaction with acids and their impact on indicators, is crucial for safe and effective utilization in various fields. Always prioritize safety when handling bases, remembering that their corrosive nature necessitates careful handling and appropriate safety measures. With a comprehensive understanding of their properties and safe handling practices, we can fully appreciate and harness the power of bases in chemistry, biology, and numerous other scientific and technological applications.
Latest Posts
Latest Posts
-
Is P Value The Same As Critical Value
Apr 08, 2025
-
Has Properties Of Both Metals And Nonmetals
Apr 08, 2025
-
Internal And External Users Of Accounting
Apr 08, 2025
-
Example Of Linear Programming In Real Life
Apr 08, 2025
-
Where In The Brain Is The Respiratory Center Located
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Is A Characteristic Of A Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.