What Is A Lone Pair In Chemistry
Muz Play
Mar 15, 2025 · 6 min read
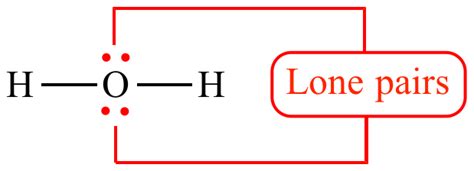
Table of Contents
What is a Lone Pair in Chemistry? A Comprehensive Guide
Understanding lone pairs is crucial for grasping fundamental concepts in chemistry, impacting everything from molecular geometry to reactivity. This comprehensive guide delves into the definition, significance, and implications of lone pairs of electrons, providing a detailed explanation for students and enthusiasts alike.
Defining Lone Pairs: The Unbonded Electrons
In chemistry, a lone pair, also known as a non-bonding pair or unshared pair, refers to a pair of valence electrons that are not involved in covalent bonding. These electrons are associated with a single atom and are not shared with another atom to form a chemical bond. Unlike bonding pairs, which are shared between two atoms, lone pairs are localized on a single atom, significantly influencing its properties and the molecule's overall behavior.
Valence Electrons and Their Role
Before diving deeper, it's essential to understand valence electrons. These are the outermost electrons in an atom, occupying the highest energy level. They are the primary players in chemical bonding, determining how an atom interacts with other atoms. The number of valence electrons an atom possesses is crucial in predicting its bonding capacity and the number of bonds it can form. Elements in the same group (vertical column) on the periodic table have the same number of valence electrons, leading to similar chemical behavior.
Visualizing Lone Pairs: Lewis Structures
Lewis structures, also known as Lewis dot diagrams, are a valuable tool for visualizing lone pairs. In a Lewis structure, valence electrons are represented as dots surrounding the element symbol. Pairs of dots represent electron pairs, and a line between two atoms represents a shared pair of electrons (a covalent bond). Lone pairs are represented as pairs of dots that are not involved in bonding.
Example: Consider the water molecule (H₂O). Oxygen has six valence electrons. Two of these electrons form covalent bonds with the two hydrogen atoms, leaving four electrons as two lone pairs on the oxygen atom. The Lewis structure of water clearly illustrates these lone pairs.
..
:O:
/ \
H H
The Significance of Lone Pairs: Impact on Molecular Properties
Lone pairs have a profound impact on a molecule's properties, influencing:
1. Molecular Geometry: VSEPR Theory
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a cornerstone of molecular geometry prediction. This theory postulates that electron pairs, both bonding and non-bonding, repel each other and arrange themselves to minimize this repulsion. Lone pairs, being closer to the nucleus, exert a stronger repulsive force than bonding pairs. This means that the presence of lone pairs significantly influences the bond angles and overall shape of the molecule.
Example: Consider methane (CH₄) and ammonia (NH₃). Methane has four bonding pairs and no lone pairs, resulting in a tetrahedral geometry with bond angles of 109.5°. Ammonia, however, has three bonding pairs and one lone pair. The lone pair exerts a stronger repulsive force, compressing the bond angles between the N-H bonds to approximately 107°. This difference in geometry directly impacts the molecule's polarity and reactivity.
2. Molecular Polarity: Dipole Moments
Lone pairs significantly contribute to a molecule's polarity. A polar molecule has a net dipole moment, meaning it possesses a positive and a negative end due to an uneven distribution of electron density. Lone pairs, by their nature, create an uneven electron distribution, contributing to the molecule's dipole moment.
Example: Water (H₂O) is a polar molecule primarily due to the presence of two lone pairs on the oxygen atom. These lone pairs create a region of higher electron density on the oxygen side, making it partially negative (δ-), while the hydrogen atoms become partially positive (δ+). This polarity is crucial for water's unique properties, like its high boiling point and excellent solvent capabilities.
3. Reactivity: Nucleophilicity and Basicity
Lone pairs play a vital role in determining a molecule's reactivity, especially its nucleophilicity and basicity. A nucleophile is a species that donates an electron pair to form a new bond. Lone pairs act as electron donors, making molecules with lone pairs potential nucleophiles. Similarly, a base is a species that accepts a proton (H⁺). Lone pairs can readily accept protons, making molecules with lone pairs potentially basic.
Example: Ammonia (NH₃) is a strong nucleophile and a weak base due to its lone pair on the nitrogen atom. This lone pair can readily donate electrons to electrophiles (electron-deficient species) and accept protons.
Lone Pairs in Different Chemical Contexts
The influence of lone pairs extends to various aspects of chemistry:
1. Organic Chemistry: Functional Groups
Lone pairs are prevalent in many organic functional groups, such as alcohols (-OH), amines (-NH₂), and ethers (-O-). These lone pairs significantly influence the reactivity and properties of these functional groups. For instance, the lone pairs on the oxygen atom in alcohols make them capable of hydrogen bonding, contributing to their higher boiling points compared to alkanes of similar molecular weight.
2. Inorganic Chemistry: Coordination Compounds
In inorganic chemistry, lone pairs play a crucial role in the formation of coordination compounds. Ligands, molecules or ions that donate electron pairs to a central metal ion, often possess lone pairs that participate in coordinate covalent bonds. The number and arrangement of lone pairs on the ligands determine the geometry and stability of the coordination complex.
3. Physical Chemistry: Spectroscopy
The presence of lone pairs can be detected and characterized using various spectroscopic techniques, such as nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy. The chemical shifts in NMR and the vibrational frequencies in IR are influenced by the electronic environment surrounding the atom, including the presence of lone pairs. These techniques allow chemists to identify and quantify lone pairs in different molecules.
Distinguishing Lone Pairs from Bonding Pairs
While both lone pairs and bonding pairs consist of two electrons, their roles and influences differ significantly. Bonding pairs are shared between two atoms, forming a covalent bond and determining the connectivity within the molecule. Lone pairs, on the other hand, are localized on a single atom, impacting the molecule's geometry, polarity, and reactivity without directly contributing to the bonding framework.
Advanced Concepts and Applications
The concept of lone pairs extends to more advanced topics in chemistry:
- Hypervalent compounds: These compounds feature atoms with more than eight electrons in their valence shell, often involving expanded octets due to the presence of lone pairs and additional bonding interactions.
- Molecular orbital theory: This theory provides a more sophisticated description of bonding and electron distribution, offering a deeper understanding of the role of lone pairs in determining molecular properties.
- Computational chemistry: Advanced computational methods allow for the precise calculation of electron density distributions, providing detailed information about the location and contribution of lone pairs.
Conclusion: The Unsung Heroes of Molecular Behavior
Lone pairs, often overlooked in introductory chemistry courses, are fundamental to understanding molecular behavior. Their influence extends from shaping molecular geometry and polarity to governing reactivity and influencing the properties of various chemical compounds. A thorough grasp of lone pairs is essential for mastering many key concepts in chemistry and advancing into more specialized areas of study. This comprehensive guide has strived to illuminate the significance of these unshared electron pairs and their pervasive influence throughout the chemical world. Further exploration of these concepts, particularly through hands-on practice with Lewis structures and VSEPR theory, will solidify your understanding and allow you to confidently predict and interpret the behavior of molecules.
Latest Posts
Latest Posts
-
Que Es La Descomposicion De Acidos
Mar 15, 2025
-
Which Factor Affects Congressional Approval Ratings The Most
Mar 15, 2025
-
Fourier Transform Of A Differential Equation
Mar 15, 2025
-
Which One Neutral Charge Proton Or Neutron
Mar 15, 2025
-
A Solids Volume And Shape Is Defintie
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is A Lone Pair In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
