What Is A Property Of An Ionic Compound
Muz Play
Mar 18, 2025 · 5 min read
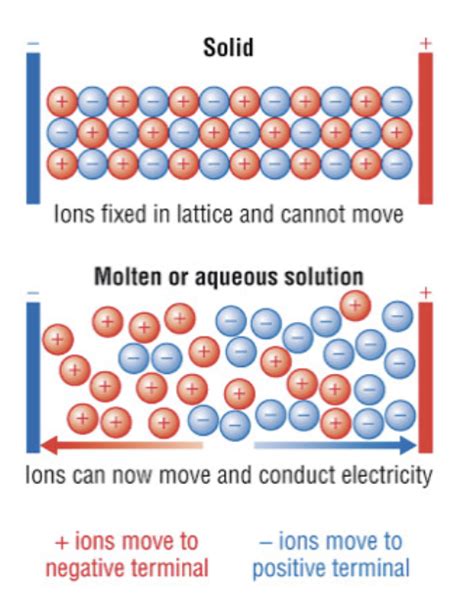
Table of Contents
Properties of Ionic Compounds: A Deep Dive
Ionic compounds, formed through the electrostatic attraction between oppositely charged ions, exhibit a unique set of properties that distinguish them from other types of compounds like covalent compounds. Understanding these properties is crucial in various fields, from chemistry and materials science to geology and medicine. This comprehensive article delves into the key characteristics of ionic compounds, explaining the underlying reasons behind their behavior.
Defining Ionic Compounds: A Foundation
Before diving into the properties, let's establish a firm understanding of what constitutes an ionic compound. Ionic compounds arise from the transfer of electrons between atoms. This transfer creates ions: positively charged cations (typically metals) and negatively charged anions (typically nonmetals). The strong electrostatic force of attraction between these oppositely charged ions forms the ionic bond, holding the compound together. This fundamental interaction dictates many of the observed properties.
Key Properties of Ionic Compounds: A Detailed Exploration
The properties of ionic compounds are directly linked to their structure and bonding. Let's examine each property in detail:
1. High Melting and Boiling Points: The Strength of Electrostatic Attraction
One of the most defining characteristics of ionic compounds is their high melting and boiling points. This stems from the strong electrostatic forces between the ions. To overcome these strong attractions and transition from a solid to a liquid (melting) or from a liquid to a gas (boiling), a significant amount of energy (heat) is required. The stronger the electrostatic forces (which depend on the charges of the ions and the distance between them), the higher the melting and boiling points. For example, sodium chloride (NaCl) has a high melting point of 801°C, reflecting the strong attraction between Na⁺ and Cl⁻ ions.
2. Crystalline Structure: Order and Arrangement
Ionic compounds typically exist as crystalline solids at room temperature. This ordered arrangement, often described as a lattice structure, maximizes the electrostatic attraction between the cations and anions. Each ion is surrounded by ions of opposite charge, creating a stable, repeating pattern. The specific arrangement depends on the size and charge of the ions involved. The regularity of this structure contributes to several other properties discussed below.
3. Hardness and Brittleness: A Delicate Balance
While ionic compounds are often hard, they are also remarkably brittle. Their hardness arises from the strong ionic bonds within the crystal lattice. However, their brittleness is a consequence of the rigid structure. When struck with a force, the layers of ions can shift, bringing ions of like charge into close proximity. This results in strong repulsive forces, causing the crystal to fracture along cleavage planes.
4. Solubility in Polar Solvents: The Role of Dipoles
Many ionic compounds are soluble in polar solvents like water. Water is a polar molecule, meaning it has a positive and a negative end due to the unequal sharing of electrons. The positive end of the water molecule is attracted to the anions in the ionic compound, and the negative end is attracted to the cations. This interaction, known as solvation, weakens the electrostatic forces holding the ionic lattice together, allowing the ions to become surrounded by water molecules and dissolve. However, not all ionic compounds are equally soluble; solubility depends on factors such as the strength of the ionic bonds and the relative strengths of the ion-solvent interactions.
5. Electrical Conductivity: Ions in Motion
Ionic compounds are typically poor conductors of electricity in the solid state. This is because the ions are fixed in their positions within the crystal lattice and cannot move freely to carry an electric current. However, when melted or dissolved in a polar solvent, ionic compounds become good conductors. In this molten or dissolved state, the ions are free to move, and the application of an electric field causes them to migrate, carrying a charge and thus enabling the flow of electricity. This property is exploited in various applications, such as electrolytic cells.
6. Electrolysis: Separating Ions
The process of electrolysis relies on the ability of molten or dissolved ionic compounds to conduct electricity. In electrolysis, an electric current is passed through the molten or dissolved ionic compound, causing the ions to migrate to the electrodes. At the electrodes, the ions undergo reduction (gain electrons) or oxidation (lose electrons), leading to the separation of the constituent elements. This is a crucial process in the extraction of metals from their ores and in various industrial applications.
7. High Density: Compact Packing
Ionic compounds often exhibit high densities compared to covalent compounds. This results from the close packing of ions in the crystal lattice. The strong electrostatic forces pull the ions together, leading to a compact arrangement with relatively little empty space. The density of an ionic compound is influenced by the size and mass of the ions involved.
Factors Affecting Properties: A Deeper Look
Several factors influence the properties of ionic compounds:
- Charge of the ions: Higher charges lead to stronger electrostatic attraction, resulting in higher melting and boiling points, and greater hardness.
- Size of the ions: Smaller ions allow for closer packing and stronger attraction, leading to higher melting and boiling points.
- Polarity of the solvent: Polar solvents are more effective at dissolving ionic compounds due to their ability to solvate the ions.
- Lattice energy: This is the energy released when gaseous ions combine to form a solid ionic compound. Higher lattice energy indicates stronger bonds and thus higher melting and boiling points.
Applications of Ionic Compounds: A Broad Spectrum
The unique properties of ionic compounds make them crucial in various applications:
- Medicine: Many ionic compounds are used as electrolytes in bodily fluids, maintaining osmotic balance. Others serve as medications or components of medications.
- Industry: They are used in fertilizers, pigments, and numerous industrial processes.
- Everyday Life: Table salt (NaCl), a common ionic compound, is a staple in our kitchens.
Conclusion: Understanding the Interplay of Structure and Properties
The properties of ionic compounds are intrinsically linked to their structure and the strong electrostatic forces between their constituent ions. Understanding these relationships is essential for predicting their behavior and utilizing their unique characteristics in various applications. From the high melting points that necessitate specialized handling to their solubility in water and electrical conductivity in solution, these compounds showcase the fascinating interplay between atomic structure and macroscopic properties. The continued study of ionic compounds remains vital in diverse scientific and technological advancements.
Latest Posts
Latest Posts
-
Which One Leaves The Solution Untouched
Mar 18, 2025
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is A Property Of An Ionic Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
