What Is A Solution And A Mixture
Muz Play
Apr 05, 2025 · 6 min read
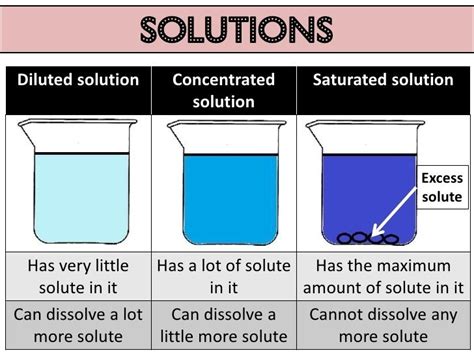
Table of Contents
What is a Solution and a Mixture? A Deep Dive into Chemistry's Building Blocks
Understanding the fundamental differences between solutions and mixtures is crucial for anyone studying chemistry, or simply wanting to grasp the basics of how matter interacts. While both involve combining different substances, their characteristics differ significantly. This detailed guide will explore the definitions, properties, and examples of solutions and mixtures, highlighting their key distinctions and practical applications. We’ll delve into the intricacies of solubility, concentration, and the various types of mixtures to build a comprehensive understanding of these essential concepts.
Defining Solutions and Mixtures
Before we dive into the specifics, let's establish clear definitions:
Mixture: A mixture is a substance composed of two or more components not chemically bonded. A key characteristic is that the components retain their individual chemical properties. Mixtures can be separated into their constituent parts using physical methods like filtration, distillation, or evaporation. Think of a salad – you can easily pick out the lettuce, tomatoes, and cucumbers. The individual ingredients haven't undergone any chemical change when combined.
Solution: A solution is a special type of homogeneous mixture. This means its composition is uniform throughout; you won't find areas richer in one component than another. A solution consists of a solute (the substance being dissolved) and a solvent (the substance doing the dissolving). Crucially, the solute and solvent are dispersed at the molecular or ionic level; they are intimately mixed. Think of salt dissolved in water – the salt disappears, but its presence is still detectable by taste and other means. The salt molecules are evenly distributed among the water molecules.
Key Differences Between Solutions and Mixtures
The following table summarizes the key differences:
| Feature | Solution | Mixture |
|---|---|---|
| Composition | Homogeneous (uniform throughout) | Can be homogeneous or heterogeneous |
| Particle Size | Solute particles are extremely small (ions or molecules) | Particle size can vary widely |
| Separation | Requires chemical methods to separate | Can be separated using physical methods |
| Solubility | Solute completely dissolves in solvent | Components may or may not dissolve completely |
| Appearance | Usually transparent | Can be transparent, translucent, or opaque |
Types of Mixtures: Beyond Solutions
While solutions represent a specific type of mixture, the broader category of mixtures encompasses a much wider range of combinations:
1. Homogeneous Mixtures:
These mixtures have a uniform composition throughout. Besides solutions, other examples include:
- Air: A mixture of gases (primarily nitrogen, oxygen, and argon).
- Saltwater (before evaporation): Though often referred to as a solution, saltwater containing undissolved solids is a homogenous mixture. A true solution is entirely transparent; the presence of sediment creates a different classification.
- Alloys: Homogeneous mixtures of metals, such as brass (copper and zinc) or bronze (copper and tin).
2. Heterogeneous Mixtures:
These mixtures have a non-uniform composition. Different regions of the mixture have different properties:
- Sand and water: You can clearly see the distinct sand particles within the water.
- Oil and water: These two liquids don't mix; they form separate layers.
- Granite: A rock composed of visible crystals of different minerals.
- Concrete: A mixture of cement, sand, gravel, and water with visibly distinct components.
- Salad: As mentioned earlier, a classic example with easily distinguishable ingredients.
Properties of Solutions: A Closer Look
Solutions possess unique properties that stem from the intimate mixing of solute and solvent at the molecular level:
- Transparency: Solutions are typically transparent, meaning light can pass through them without significant scattering.
- Filtration: Solutions cannot be separated by simple filtration because the solute particles are too small to be trapped by filter paper.
- Homogeneity: The composition is uniform throughout. A sample taken from any part of the solution will have the same concentration.
- Solubility: The maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure is its solubility. This property is crucial in determining the concentration of a solution.
Factors Affecting Solubility
Several factors influence how much solute can dissolve in a solvent:
- Temperature: Generally, increasing the temperature increases the solubility of solids in liquids. However, the effect of temperature on gas solubility is the opposite; increased temperature reduces gas solubility.
- Pressure: Pressure primarily affects the solubility of gases in liquids. Increasing the pressure increases the solubility of gases. This is Henry's Law.
- Nature of the solute and solvent: "Like dissolves like" is a common rule of thumb. Polar solvents (like water) tend to dissolve polar solutes (like sugar), while nonpolar solvents (like oil) dissolve nonpolar solutes (like fats).
- Particle size: Smaller solute particles dissolve faster than larger ones due to increased surface area.
Expressing Concentration in Solutions
The concentration of a solution indicates the amount of solute present in a given amount of solvent or solution. Several ways exist to express concentration:
- Molarity (M): Moles of solute per liter of solution.
- Molality (m): Moles of solute per kilogram of solvent.
- Percent by mass (% w/w): Grams of solute per 100 grams of solution.
- Percent by volume (% v/v): Milliliters of solute per 100 milliliters of solution.
- Parts per million (ppm): A way to express very low concentrations.
Applications of Solutions and Mixtures
Solutions and mixtures play vital roles in various fields:
- Medicine: Many medicines are solutions or mixtures, allowing for precise dosage and efficient drug delivery. Intravenous solutions deliver fluids and nutrients directly into the bloodstream.
- Industry: Solutions and mixtures are used in countless industrial processes, including manufacturing, metal refining, and chemical synthesis. Alloys, for example, are crucial in construction and engineering.
- Agriculture: Fertilizers are mixtures of various nutrients dissolved in water to provide plants with essential elements.
- Food science: Many food products are solutions or mixtures, from soft drinks to sauces to baked goods.
- Environmental science: Understanding the properties of solutions and mixtures is crucial for managing pollution and studying environmental processes. The composition of water bodies is a complex mixture.
Advanced Concepts: Colloids and Suspensions
While solutions, mixtures and the differences between them are fundamental to chemical understanding, it is important to note that some mixtures exist between the clearly defined boundaries.
Colloids are mixtures where one substance is dispersed evenly throughout another. Unlike solutions, these dispersed particles are larger but not large enough to settle out of the mixture. Examples include milk, fog, and paint. The particles in a colloid are typically between 1 and 1000 nanometers in size, a range significantly larger than those in a solution. They exhibit the Tyndall effect, scattering light, resulting in a cloudy appearance.
Suspensions are heterogeneous mixtures where solid particles are dispersed in a liquid. The particles are much larger than those in solutions or colloids and will settle out over time if left undisturbed. Examples include muddy water or a mixture of sand and water.
Conclusion
The distinction between solutions and mixtures is a fundamental concept in chemistry. While both involve combining multiple substances, solutions represent a specific type of homogeneous mixture characterized by uniform composition, molecular-level dispersion of solute particles, and transparency. Mixtures, on the other hand, encompass a wider range of combinations, including both homogeneous and heterogeneous types. Understanding the properties, types, and applications of solutions and mixtures is essential for various scientific and practical applications, from medicine and industry to environmental science and food production. The further distinction between colloids and suspensions helps to refine our understanding of the spectrum between true solutions and more heterogeneous dispersions. This comprehensive overview provides a solid foundation for further exploration of these crucial chemical concepts.
Latest Posts
Latest Posts
-
Used To Help Substances Enter Or Exit The Cell Membrane
Apr 05, 2025
-
Verify That Is A Solution To The Differential Equation
Apr 05, 2025
-
Gaining Or Losing Electrons Is Called
Apr 05, 2025
-
How Sociology Differs From Other Social Sciences
Apr 05, 2025
-
Do Ionic Compounds Have High Boiling Points
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about What Is A Solution And A Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
