What Is A Solution In Biology
Muz Play
Mar 24, 2025 · 6 min read
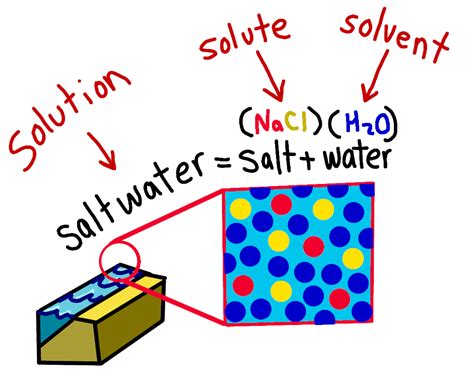
Table of Contents
What is a Solution in Biology? A Deep Dive into Solutes, Solvents, and Cellular Processes
In the biological world, the concept of a solution is fundamental to understanding how life functions at a molecular level. From the simplest single-celled organisms to complex multicellular beings, solutions play a critical role in numerous processes, impacting everything from cellular transport to enzymatic reactions. This article will delve deep into the definition of a solution in biology, explore the key components, discuss various types of solutions, and examine their significance in different biological contexts.
Defining a Solution in Biological Terms
A solution, in biology, is a homogeneous mixture composed of two or more substances. These substances are uniformly dispersed at a molecular level, meaning that the components cannot be easily separated by physical methods like filtration. This contrasts with suspensions, where particles are larger and can be separated. Crucially, in a solution, the dissolved substance is not chemically altered.
The key components of a biological solution are:
-
Solvent: This is the substance that dissolves other substances. In biological systems, the most common solvent is water (H₂O), owing to its unique properties like polarity and high dielectric constant. Water's polarity allows it to interact with and dissolve many polar and ionic substances. This is why water is often referred to as the "universal solvent."
-
Solute: This is the substance that is dissolved in the solvent. Solutes can be various molecules, including ions, sugars, proteins, gases (like oxygen and carbon dioxide), and many other organic and inorganic compounds. The concentration of the solute determines the solution's properties.
The combination of solute and solvent forms a solution, and the ratio between them defines the solution's concentration. This concentration is frequently expressed as molarity (moles of solute per liter of solution), but other units like percentage concentration or molality are also used depending on the context.
Types of Solutions in Biology
Solutions in biological systems can be categorized in several ways:
Based on the Polarity of the Solute and Solvent:
-
Polar Solutions: These solutions consist of polar solvents (like water) and polar solutes. Polar molecules possess an uneven distribution of charge, creating partial positive and negative regions. The attraction between the polar solvent and solute molecules leads to their dissolution. Many biological molecules, including sugars and amino acids, are polar and dissolve readily in water.
-
Nonpolar Solutions: These solutions involve nonpolar solvents (like lipids) and nonpolar solutes. Nonpolar molecules have an even distribution of charge and interact primarily through weak van der Waals forces. Lipids, for instance, are nonpolar and dissolve in other lipids to form solutions like cell membranes.
-
Mixed Solutions: Many biological solutions are complex mixtures involving both polar and nonpolar components. For example, cell cytoplasm contains a mixture of water, ions, proteins, and lipids. The interaction between these components is crucial for maintaining cell structure and function.
Based on the Concentration of the Solute:
-
Isotonic Solutions: These solutions have the same concentration of solutes as the surrounding environment. Cells placed in isotonic solutions maintain their shape and size because there is no net movement of water across the cell membrane.
-
Hypertonic Solutions: These solutions have a higher concentration of solutes than the surrounding environment. Cells placed in hypertonic solutions will lose water through osmosis, leading to shrinkage or crenation.
-
Hypotonic Solutions: These solutions have a lower concentration of solutes than the surrounding environment. Cells placed in hypotonic solutions will gain water through osmosis, potentially leading to swelling and lysis (cell bursting).
Based on the State of Matter:
-
Liquid Solutions: The most common type in biological systems, where the solvent is a liquid (usually water).
-
Gaseous Solutions: Gases dissolved in liquids are also relevant in biology, for example, oxygen dissolved in blood plasma.
-
Solid Solutions: While less frequent, solid solutions exist in certain biological contexts, such as the incorporation of certain minerals into bone tissue.
The Significance of Solutions in Biological Processes
Solutions are crucial to numerous biological processes, including:
1. Cellular Transport:
The movement of substances across cell membranes is fundamentally linked to solutions. Processes like osmosis (movement of water across a selectively permeable membrane) and diffusion (movement of solutes down a concentration gradient) rely on the presence of solutions. Understanding the tonicity of a solution is critical for predicting the effects on cells.
2. Enzyme Activity:
Enzymes, the biological catalysts that accelerate chemical reactions, often require specific ionic environments to function optimally. The concentration of ions in solution directly affects enzyme activity and plays a critical role in metabolic pathways. Many enzyme reactions occur within a specific pH range, which is directly determined by the solution’s properties.
3. Blood and Body Fluids:
Blood plasma is a complex solution containing various dissolved substances, including glucose, amino acids, ions, hormones, and gases. The precise composition of these solutions is vital for maintaining homeostasis, the stable internal environment necessary for life. Other bodily fluids, like interstitial fluid and lymph, are also solutions crucial for nutrient transport and waste removal.
4. Photosynthesis and Respiration:
Photosynthesis, the process by which plants convert light energy into chemical energy, and cellular respiration, the process of energy release from glucose, both involve solutions. The availability of dissolved CO₂ and O₂ in cellular solutions is critical for these processes to occur efficiently. Likewise, the movement of water and ions in plant tissues directly impacts their photosynthetic capabilities.
5. Nutrient Uptake in Plants:
Plants absorb nutrients dissolved in the soil water. The concentration of essential nutrients in the soil solution directly affects plant growth and health. The availability of water and dissolved minerals in the soil solution determines the efficiency of nutrient uptake by plant roots.
Maintaining Solution Balance: Homeostasis
Maintaining the appropriate concentration and composition of solutions in biological systems is essential for survival. This is achieved through complex regulatory mechanisms that contribute to homeostasis. These mechanisms involve:
-
Osmosis and Water Regulation: The kidneys play a crucial role in maintaining water balance by regulating the excretion of water and solutes.
-
Ion Channels and Pumps: Cells possess specialized channels and pumps that control the movement of ions across their membranes, maintaining the correct internal ion concentrations.
-
Buffering Systems: These systems help to maintain a stable pH in biological solutions by resisting changes in acidity or alkalinity. The bicarbonate buffer system is a critical example, maintaining blood pH within a narrow range.
Applications and Further Research
Understanding solutions in biology has wide-ranging applications, impacting fields such as:
-
Medicine: Intravenous fluids must have the correct tonicity to avoid harming cells. Drug delivery often relies on the solubility of the drug in biological solutions.
-
Agriculture: Soil science relies heavily on understanding the properties of soil solutions and their impact on plant growth.
-
Environmental Science: Water quality assessments involve measuring the concentration of various dissolved substances in water bodies.
-
Biotechnology: Many biotechnological processes involve working with solutions containing enzymes, proteins, and other biomolecules.
Ongoing research continues to refine our understanding of solution behavior in biological systems. For example, researchers are investigating the complex interactions between solutes and solvents in crowded cellular environments, where macromolecules occupy a significant volume. Further research will undoubtedly uncover more insights into the intricate roles solutions play in maintaining life.
Conclusion
In conclusion, the concept of a solution in biology is a cornerstone of understanding the intricate molecular processes that underpin life. From the simplest transport of substances across membranes to the complex regulatory mechanisms maintaining homeostasis, solutions are integral to biological function. The composition and concentration of solutions profoundly influence biological systems, making the study of solutions crucial for various scientific disciplines, from medicine and agriculture to environmental science and biotechnology. Further research in this area is vital for unlocking deeper insights into the wonders of life itself.
Latest Posts
Latest Posts
-
Active Transport Must Function Continuously Because
Mar 26, 2025
-
Chemical Equilibrium And Le Chateliers Principle Lab Answers
Mar 26, 2025
-
What Is The Function Of Channel Proteins
Mar 26, 2025
-
What Happens To A Plant Cell In Hypertonic Solution
Mar 26, 2025
-
Atomic Mass Equals The Number Of
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is A Solution In Biology . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
