What Happens To A Plant Cell In Hypertonic Solution
Muz Play
Mar 26, 2025 · 6 min read
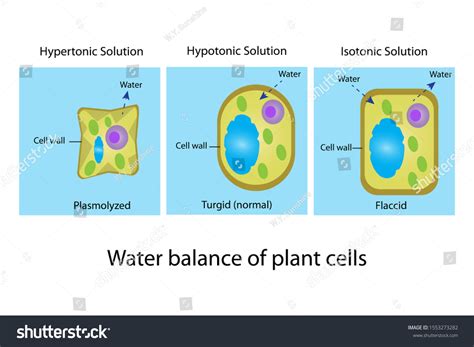
Table of Contents
- What Happens To A Plant Cell In Hypertonic Solution
- Table of Contents
- What Happens to a Plant Cell in a Hypertonic Solution?
- Understanding Osmosis and Tonicity
- The Plant Cell Structure: A Crucial Factor
- What Happens When a Plant Cell is Placed in a Hypertonic Solution?
- Factors Influencing Plasmolysis
- Reversal of Plasmolysis: Deplasmolysis
- Practical Applications and Significance
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
What Happens to a Plant Cell in a Hypertonic Solution?
Plant cells, unlike animal cells, possess a rigid cell wall that plays a crucial role in their response to different osmotic environments. Understanding how a plant cell behaves in a hypertonic solution is fundamental to grasping plant physiology and its implications in various fields like agriculture and horticulture. This comprehensive article will delve into the intricate processes that occur when a plant cell is placed in a hypertonic solution, exploring the underlying mechanisms and consequences.
Understanding Osmosis and Tonicity
Before we examine the specific effects on plant cells, let's briefly review the concepts of osmosis and tonicity. Osmosis is the passive movement of water across a selectively permeable membrane from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration). This movement continues until equilibrium is reached, or the water potential is equal on both sides of the membrane.
Tonicity refers to the relative concentration of solutes in two solutions separated by a selectively permeable membrane. Three main types of tonicity exist:
- Hypotonic: The solution has a lower solute concentration than the cell's cytoplasm. Water moves into the cell.
- Isotonic: The solution has the same solute concentration as the cell's cytoplasm. There is no net movement of water.
- Hypertonic: The solution has a higher solute concentration than the cell's cytoplasm. Water moves out of the cell.
It's the hypertonic environment that we'll be focusing on in this article concerning plant cells.
The Plant Cell Structure: A Crucial Factor
The structural components of a plant cell are essential in determining its response to a hypertonic solution. These include:
- Cell Wall: A rigid outer layer composed primarily of cellulose, providing structural support and protection. Its permeability allows water and certain solutes to pass through.
- Cell Membrane (Plasma Membrane): A selectively permeable membrane that regulates the movement of substances into and out of the cell. It is crucial in the osmotic process.
- Vacuole: A large, central vacuole occupies a significant portion of the plant cell's volume. It's filled with cell sap, a solution containing various ions, sugars, and other substances. The vacuole plays a vital role in maintaining turgor pressure.
- Cytoplasm: The jelly-like substance filling the cell, containing various organelles and participating in cellular processes.
What Happens When a Plant Cell is Placed in a Hypertonic Solution?
When a plant cell is immersed in a hypertonic solution, the water potential outside the cell is lower than inside the cell. This creates a concentration gradient that drives the movement of water across the cell membrane. This is where the critical differences between plant and animal cells become apparent.
-
Water Loss from the Cytoplasm: Water moves out of the cytoplasm and the vacuole via osmosis, across the selectively permeable cell membrane. This outward movement is driven by the attempt to equalize the water potential across the membrane.
-
Plasmolysis: As water exits the cell, the vacuole shrinks, reducing the turgor pressure (the pressure exerted by the cell contents against the cell wall). This leads to plasmolysis, a process where the cell membrane pulls away from the cell wall. This detachment is particularly visible at the corners of the cell. The cell becomes flaccid and loses its rigidity. The degree of plasmolysis depends on the concentration of the hypertonic solution and the duration of exposure.
-
Cell Wall Remains Intact: Unlike animal cells that might burst in a hypotonic solution, the rigid cell wall prevents the plant cell from collapsing completely, even under extreme plasmolysis. The cell wall provides structural support, preventing catastrophic cell damage.
-
Metabolic Changes: The loss of turgor pressure and the altered cytoplasmic environment can impact various cellular processes. Metabolic activity may slow down or alter. The cell's ability to transport substances, conduct photosynthesis, and maintain its overall function can be compromised.
-
Potential for Cell Death: Prolonged exposure to a hypertonic solution can lead to irreversible damage and ultimately, cell death. If the water loss is severe, the cell may become irreversibly plasmolyzed, affecting its viability.
Factors Influencing Plasmolysis
Several factors influence the extent and rate of plasmolysis in plant cells:
- Concentration of the Hypertonic Solution: A higher concentration of solutes in the hypertonic solution will lead to a greater water loss and more pronounced plasmolysis.
- Duration of Exposure: Longer exposure to a hypertonic solution intensifies the effects of plasmolysis.
- Plant Species: Different plant species have varying degrees of tolerance to hypertonic stress due to differences in cell wall composition, membrane permeability and water regulation mechanisms.
- Cell Age: Younger cells generally have thinner cell walls and may be more susceptible to plasmolysis.
Reversal of Plasmolysis: Deplasmolysis
If the plant cell is transferred from a hypertonic solution to a hypotonic solution or an isotonic solution, deplasmolysis can occur. This is the process where the cell regains its turgor pressure as water moves back into the cell. The cell membrane reattaches to the cell wall, and the cell returns to its normal, turgid state. The speed of deplasmolysis depends on the concentration gradient and other factors.
Practical Applications and Significance
Understanding the effects of hypertonic solutions on plant cells has significant implications in various fields:
-
Agriculture: Managing soil salinity is crucial for successful crop production. High salt concentrations in the soil can create hypertonic conditions for plant roots, leading to reduced water uptake and growth. Understanding these mechanisms helps develop strategies for salinity tolerance in crops.
-
Horticulture: Maintaining appropriate osmotic conditions is important for plant tissue culture and propagation. Understanding plasmolysis allows for the optimization of culture media to ensure optimal cell growth and development.
-
Food Preservation: Hypertonic solutions, such as concentrated sugar or salt solutions, are often used in food preservation. These solutions create hypertonic conditions that draw water out of microorganisms, inhibiting their growth and preventing spoilage.
-
Plant Biology Research: Studying plasmolysis helps researchers understand the mechanisms of water transport and regulation in plants, leading to advancements in plant biotechnology and genetic engineering for improved crop yields and stress tolerance.
Conclusion
The response of a plant cell to a hypertonic solution is a complex process involving the intricate interplay of osmosis, the cell's structural components, and various cellular processes. Plasmolysis, characterized by water loss, shrinkage of the vacuole, and detachment of the cell membrane from the cell wall, is a crucial consequence. While the rigid cell wall prevents complete cell collapse, prolonged exposure can lead to irreversible damage and cell death. Understanding this phenomenon is vital for advancements in various fields, from agriculture and horticulture to food preservation and plant biology research. The knowledge gained from studying plasmolysis allows us to develop strategies to mitigate the effects of hypertonic stress and enhance plant resilience and productivity. Further research continues to unravel the intricate details of these processes, paving the way for improved crop yields, stress-tolerant varieties, and a better understanding of the fundamental mechanisms that govern plant life.
Latest Posts
Latest Posts
-
Critical Value Of 99 Confidence Interval
Mar 29, 2025
-
The Monomers Of Proteins Are Called
Mar 29, 2025
-
Solving Exponential Equations Using Logarithms Worksheet
Mar 29, 2025
-
What Is The Relationship Between Concentration And Absorbance
Mar 29, 2025
-
Finding The Molarity Of A Titration
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Happens To A Plant Cell In Hypertonic Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
