What Is The Relationship Between Concentration And Absorbance
Muz Play
Mar 29, 2025 · 7 min read
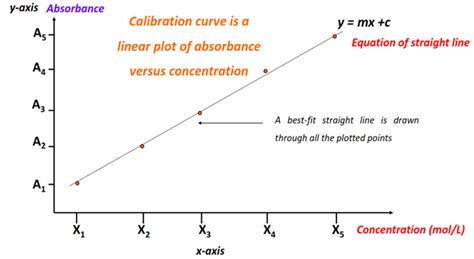
Table of Contents
The Intimate Relationship Between Concentration and Absorbance: A Deep Dive into Beer-Lambert Law
Understanding the relationship between concentration and absorbance is fundamental in various scientific fields, from analytical chemistry and biochemistry to environmental science and materials science. This relationship is elegantly described by the Beer-Lambert Law, a cornerstone of spectrophotometry. This comprehensive guide will explore this relationship in detail, explaining the underlying principles, practical applications, and limitations of the Beer-Lambert Law.
Understanding the Beer-Lambert Law: The Foundation of Spectrophotometry
The Beer-Lambert Law, also known as the Beer-Lambert-Bouguer Law, is a mathematical expression that quantifies the relationship between the absorbance of light by a solution and the concentration of the analyte within that solution. The law states that the absorbance of a solution is directly proportional to both the concentration of the absorbing species and the path length of the light through the solution.
The equation representing the Beer-Lambert Law is:
A = εbc
Where:
- A represents the absorbance of the solution (unitless). Absorbance is a logarithmic measure of the transmitted light's intensity relative to the incident light's intensity.
- ε represents the molar absorptivity (or molar extinction coefficient) – a constant specific to the absorbing species and the wavelength of light used (L mol⁻¹ cm⁻¹). It reflects how strongly a substance absorbs light at a particular wavelength. Higher molar absorptivity means stronger absorption.
- b represents the path length of the light through the solution – the distance the light travels through the sample (typically expressed in centimeters, cm). This is usually the width of the cuvette used in the spectrophotometer.
- c represents the concentration of the absorbing species in the solution (typically expressed in moles per liter, mol L⁻¹ or M).
This equation shows the direct proportionality: if you double the concentration (c), you double the absorbance (A), assuming ε and b remain constant. Similarly, doubling the path length (b) will also double the absorbance.
What is Absorbance?
Absorbance is not simply how much light is blocked; it's a logarithmic measure of the light's intensity reduction as it passes through the sample. It’s defined as:
A = log₁₀(I₀/I)
Where:
- I₀ is the intensity of the incident light (the light entering the sample).
- I is the intensity of the transmitted light (the light that passes through the sample).
A higher absorbance value indicates that a greater proportion of the incident light is absorbed by the sample.
What is Molar Absorptivity (ε)?
Molar absorptivity (ε) is a crucial parameter in the Beer-Lambert Law. It's a measure of how strongly a substance absorbs light at a specific wavelength. It's an intrinsic property of the substance and the wavelength, meaning it remains constant under specific conditions (temperature, solvent, etc.). Different substances have different molar absorptivities, and even for the same substance, the molar absorptivity varies with the wavelength of light. This variation is often represented by an absorption spectrum – a graph plotting absorbance against wavelength.
Practical Applications of the Beer-Lambert Law: Utilizing the Concentration-Absorbance Relationship
The Beer-Lambert Law finds widespread application in various fields, making it a powerful tool for quantitative analysis. Here are some key examples:
1. Quantitative Analysis in Chemistry:
The most common application is determining the concentration of an unknown solution. By measuring the absorbance of the solution at a specific wavelength where the analyte strongly absorbs, and knowing the molar absorptivity (ε) and path length (b), the concentration (c) can be calculated directly using the Beer-Lambert equation. This is especially useful in:
- Analytical Chemistry: Determining the concentration of various ions, compounds, or molecules in solutions.
- Clinical Chemistry: Measuring the concentration of metabolites, hormones, or drugs in biological samples like blood or urine.
- Environmental Monitoring: Assessing the concentration of pollutants in water or air samples.
2. Spectrophotometry:
Spectrophotometry, a technique that measures the absorbance or transmission of light through a solution, heavily relies on the Beer-Lambert Law. Spectrophotometers are widely used instruments in various labs, allowing for precise measurement of absorbance at specific wavelengths. This facilitates quantitative analysis and the study of reaction kinetics, allowing scientists to monitor the change in concentration of reactants or products over time.
3. Biochemistry and Molecular Biology:
In biochemistry and molecular biology, the Beer-Lambert Law is crucial for:
- Protein Quantification: Determining the concentration of proteins in solutions using methods like the Bradford assay or Lowry assay, which rely on the absorbance of colorimetric reagents bound to the protein.
- Enzyme Kinetics: Studying the kinetics of enzyme-catalyzed reactions by monitoring the change in absorbance of substrates or products over time.
- DNA and RNA Quantification: Measuring the concentration of nucleic acids using UV spectrophotometry at 260 nm.
4. Materials Science:
The Beer-Lambert Law also finds application in materials science, particularly in the characterization of materials' optical properties. It is used to determine the concentration of dopants in semiconductor materials or to study the absorption characteristics of different materials.
Limitations and Deviations from the Beer-Lambert Law: Understanding the Exceptions
While the Beer-Lambert Law is a powerful tool, it's crucial to understand its limitations and situations where it may not accurately predict absorbance. Several factors can cause deviations from linearity:
1. High Concentrations:
At high concentrations, the interactions between analyte molecules can alter the analyte's absorbance properties, leading to deviations from linearity. This is because the molecules may interact with each other, affecting their ability to absorb light individually.
2. Chemical Changes:
If the analyte undergoes chemical changes (e.g., dissociation, association, or reaction with the solvent), the absorbance may deviate from linearity because the absorbing species' concentration is not accurately represented.
3. Stray Light:
Stray light refers to light that reaches the detector without passing through the sample. This can significantly affect absorbance measurements, especially at high absorbance values. Stray light leads to underestimation of absorbance, especially when working with highly concentrated samples.
4. Non-monochromatic Light:
The Beer-Lambert Law assumes monochromatic light (light of a single wavelength). If the light source is not perfectly monochromatic, the molar absorptivity (ε) may vary across the wavelengths present in the beam, causing deviations from linearity.
5. Fluorescence and Phosphorescence:
These phenomena occur when the analyte emits light after absorbing light. These emissions can interfere with the measured absorbance values, affecting the accuracy of the Beer-Lambert Law.
6. Scattering:
Light scattering by the sample can lead to reduced transmission of light, mimicking absorption and causing deviations from the Beer-Lambert Law. This is especially true for turbid or particulate samples.
Strategies for Minimizing Deviations and Ensuring Accurate Measurements
To ensure accurate measurements and minimize deviations from the Beer-Lambert Law, several strategies can be employed:
- Using dilute solutions: Working with dilute solutions minimizes intermolecular interactions that lead to deviations at high concentrations.
- Using monochromatic light: Employing a light source that provides highly monochromatic light minimizes errors arising from variations in molar absorptivity at different wavelengths.
- Employing appropriate cuvettes: Using clean, matched cuvettes ensures consistent path length and minimizes stray light effects.
- Correcting for stray light: Some spectrophotometers have built-in corrections for stray light.
- Employing appropriate solvent: Using an appropriate solvent ensures that the analyte remains stable and prevents unwanted chemical changes.
- Using a calibration curve: Creating a calibration curve by measuring absorbance for solutions with known concentrations helps determine whether the Beer-Lambert Law is followed for the analyte in the range of concentrations of interest. A calibration curve can account for deviations from linearity.
Conclusion: The Beer-Lambert Law – A Powerful, Yet Imperfect Tool
The Beer-Lambert Law provides a fundamental and essential relationship between concentration and absorbance, forming the bedrock of quantitative analysis using spectrophotometry. Its wide applicability in diverse fields underscores its importance. However, it is crucial to be aware of its limitations and potential deviations. By understanding these limitations and employing appropriate experimental techniques, scientists can harness the power of the Beer-Lambert Law for precise and reliable quantitative measurements. Careful experimental design, proper sample preparation, and awareness of potential sources of error are critical for obtaining meaningful results and applying this law effectively. Always consider the potential limitations and strive for optimal experimental conditions to ensure accurate and reliable results when using the Beer-Lambert Law in your analysis.
Latest Posts
Latest Posts
-
What Are The Factors Affecting The Rate Of Diffusion
Apr 01, 2025
-
What Is The Vsepr Geometry Of The Particle
Apr 01, 2025
-
How To Calculate Current Through Each Resistor
Apr 01, 2025
-
Barriers To Entry For Monopolistic Competition
Apr 01, 2025
-
The Most Reactive Metals Are The
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Relationship Between Concentration And Absorbance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
