What Is An Ionic Compound Made Of Metal And Nonmetal
Muz Play
Mar 19, 2025 · 6 min read
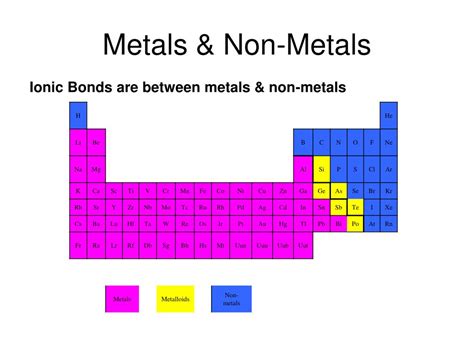
Table of Contents
What is an Ionic Compound Made of Metal and Nonmetal?
Ionic compounds are fundamental substances in chemistry, forming the basis for many materials we encounter daily. Understanding their composition and properties is crucial for comprehending various chemical processes and applications. This in-depth article explores the world of ionic compounds, focusing specifically on those formed from the union of metals and nonmetals. We will delve into the intricacies of their formation, properties, and practical significance.
The Building Blocks: Metals and Nonmetals
Before understanding ionic compounds, let's establish a clear picture of their constituent elements: metals and nonmetals.
Metals: The Generous Electron Donors
Metals, located on the left side of the periodic table, are characterized by their relatively low electronegativity. This means they readily lose electrons, becoming positively charged ions called cations. Their metallic bonding, a sea of delocalized electrons, accounts for their characteristic properties like malleability, ductility, and excellent conductivity of heat and electricity. Examples of common metals include sodium (Na), magnesium (Mg), iron (Fe), and copper (Cu). The number of electrons a metal atom loses typically corresponds to its group number on the periodic table (with some exceptions for transition metals).
Nonmetals: The Electron Acceptors
Nonmetals, situated on the right side of the periodic table, exhibit high electronegativity. This high electronegativity signifies their strong attraction for electrons. Consequently, they tend to gain electrons, forming negatively charged ions called anions. Nonmetals often exist as molecules (like oxygen gas, O₂) or in various allotropic forms (like carbon as diamond or graphite). Examples include chlorine (Cl), oxygen (O), sulfur (S), and nitrogen (N). The number of electrons a nonmetal atom gains is often determined by the number of electrons needed to achieve a stable octet (eight electrons in its valence shell).
The Ionic Bond: An Electrostatic Attraction
The heart of an ionic compound lies in the ionic bond. This bond is formed through the electrostatic attraction between oppositely charged ions: the positively charged metal cation and the negatively charged nonmetal anion. This attraction is incredibly strong, resulting in the formation of a crystalline lattice structure.
The Formation of an Ionic Bond: A Step-by-Step Process
-
Electron Transfer: The process begins with the transfer of one or more electrons from the metal atom to the nonmetal atom. The metal atom, losing electrons, becomes a positively charged cation. The nonmetal atom, gaining electrons, becomes a negatively charged anion.
-
Electrostatic Attraction: The oppositely charged ions are then drawn together by a strong electrostatic force, forming the ionic bond. This attraction isn't a sharing of electrons like in covalent bonds; it's a purely electrostatic interaction.
-
Crystal Lattice Formation: The ions arrange themselves in a highly ordered, three-dimensional structure called a crystal lattice. This lattice structure maximizes the attractive forces between the oppositely charged ions while minimizing the repulsive forces between ions of the same charge. The specific arrangement of ions in the lattice depends on the size and charge of the ions involved.
Properties of Ionic Compounds
The strong electrostatic forces within the crystal lattice impart distinct properties to ionic compounds:
-
High Melting and Boiling Points: The strong ionic bonds require significant energy to break, resulting in high melting and boiling points. This is in contrast to covalent compounds, which generally have lower melting and boiling points.
-
Brittle Nature: Ionic crystals are brittle because a slight shift in the crystal lattice can cause like-charged ions to come into close proximity, leading to strong repulsive forces and causing the crystal to shatter.
-
Solubility in Water: Many ionic compounds are soluble in water. Water molecules, being polar, can surround and interact with the charged ions, effectively pulling them apart and dissolving the crystal lattice.
-
Electrical Conductivity: Ionic compounds generally do not conduct electricity in their solid state because the ions are held tightly in the crystal lattice and cannot move freely. However, they become excellent conductors when molten (melted) or dissolved in water, as the ions are then free to move and carry an electric current.
Examples of Ionic Compounds
Let's examine some common examples of ionic compounds formed from metals and nonmetals:
-
Sodium Chloride (NaCl): Common table salt, formed from the reaction of sodium (Na), a metal, and chlorine (Cl), a nonmetal. Sodium loses one electron to become Na⁺, and chlorine gains one electron to become Cl⁻.
-
Magnesium Oxide (MgO): Magnesium (Mg), a metal, loses two electrons to form Mg²⁺, while oxygen (O), a nonmetal, gains two electrons to form O²⁻.
-
Potassium Iodide (KI): Potassium (K), a metal, loses one electron to become K⁺, and iodine (I), a nonmetal, gains one electron to become I⁻.
-
Calcium Chloride (CaCl₂): Calcium (Ca), a metal, loses two electrons to form Ca²⁺, and each chlorine atom (Cl), a nonmetal, gains one electron to become Cl⁻. Thus, two chlorine atoms are needed to balance the charge of one calcium ion.
-
Aluminum Oxide (Al₂O₃): Aluminum (Al), a metal, loses three electrons to form Al³⁺, and each oxygen atom (O), a nonmetal, gains two electrons to become O²⁻. The ratio of aluminum to oxygen is 2:3 to balance the charges.
Naming Ionic Compounds
The naming of ionic compounds follows a systematic approach:
-
Cation (Metal) Name: The name of the metal cation is written first. If the metal can form multiple ions (like iron, Fe²⁺ or Fe³⁺), the charge is indicated using Roman numerals in parentheses (e.g., iron(II) or iron(III)).
-
Anion (Nonmetal) Name: The name of the nonmetal anion is written second, with the ending changed to "-ide" (e.g., chlorine becomes chloride, oxygen becomes oxide, sulfur becomes sulfide).
For example:
- NaCl: Sodium chloride
- MgO: Magnesium oxide
- KI: Potassium iodide
- CaCl₂: Calcium chloride
- Al₂O₃: Aluminum oxide
Applications of Ionic Compounds
Ionic compounds are ubiquitous, finding applications in numerous fields:
-
Medicine: Many ionic compounds are essential for human health, serving as electrolytes (like sodium chloride), or being incorporated into pharmaceuticals.
-
Agriculture: Ionic compounds like fertilizers (containing nitrogen, phosphorus, and potassium) are crucial for plant growth.
-
Industry: Ionic compounds are used extensively in various industrial processes, including the production of metals, ceramics, and various chemicals.
-
Construction: Many building materials incorporate ionic compounds for their strength and durability.
-
Food Science: Sodium chloride (salt) is a crucial flavor enhancer and preservative in food.
Beyond the Basics: Polyatomic Ions
While the examples above focused on simple monatomic ions, many ionic compounds involve polyatomic ions. These are ions composed of two or more atoms covalently bonded together and carrying an overall charge. Common polyatomic ions include:
- Nitrate (NO₃⁻): Found in fertilizers and explosives.
- Sulfate (SO₄²⁻): A component of many minerals and acids.
- Phosphate (PO₄³⁻): Essential for biological processes and fertilizers.
- Ammonium (NH₄⁺): Used in fertilizers and cleaning agents.
The naming of ionic compounds containing polyatomic ions follows similar principles, using the name of the polyatomic ion directly. For example, sodium nitrate (NaNO₃), ammonium sulfate ((NH₄)₂SO₄), and calcium phosphate (Ca₃(PO₄)₂)
Conclusion
Ionic compounds, formed from the electrostatic attraction between metal cations and nonmetal anions, are a cornerstone of chemistry and play vital roles in various aspects of our lives. Their distinct properties, stemming from their crystal lattice structure and strong ionic bonds, make them indispensable in medicine, agriculture, industry, and everyday life. A comprehensive understanding of their formation, properties, and naming conventions is crucial for anyone pursuing studies or careers in science and related fields. This knowledge lays the foundation for comprehending more complex chemical systems and phenomena.
Latest Posts
Latest Posts
-
How To Identify Weak And Strong Acids And Bases
Mar 19, 2025
-
Do Protons Have About The Same Mass As Neutrons
Mar 19, 2025
-
What Is Monomer Of Nucleic Acids
Mar 19, 2025
-
Inverted Vs Everted Palindromic Dna Sequence Example
Mar 19, 2025
-
What Makes Up Most Of The Mass Of An Atom
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is An Ionic Compound Made Of Metal And Nonmetal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
