What Is Atomic Number Of Oxygen
Muz Play
Mar 21, 2025 · 5 min read
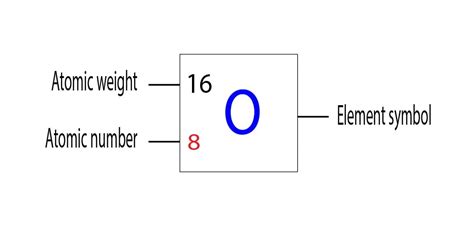
Table of Contents
What is the Atomic Number of Oxygen? A Deep Dive into the Element's Properties and Significance
Oxygen, the life-giving element, is ubiquitous in our world. But what truly defines this crucial component of our atmosphere and ourselves? The answer lies in its atomic number, a fundamental property that dictates its chemical behavior and physical characteristics. This comprehensive article delves deep into the atomic number of oxygen, exploring its implications and the broader context of atomic structure.
Understanding Atomic Number: The Identity of an Element
The atomic number of an element is essentially its unique identifier. It represents the number of protons found in the nucleus of a single atom of that element. This number is crucial because:
-
It defines the element: Every element possesses a unique atomic number. No two elements share the same number of protons. For instance, an atom with one proton is hydrogen, an atom with two is helium, and so on.
-
It determines the chemical properties: The number of protons dictates the number of electrons in a neutral atom (equal to the number of protons). These electrons are arranged in shells or energy levels surrounding the nucleus, and their configuration determines how the atom will interact with other atoms – its chemical properties.
-
It’s foundational to the periodic table: The periodic table arranges elements in order of increasing atomic number, reflecting their underlying structural similarities and periodic trends in their properties.
The Atomic Number of Oxygen: 8
The atomic number of oxygen is 8. This means that every oxygen atom contains eight protons in its nucleus. In a neutral oxygen atom, it also contains eight electrons orbiting the nucleus. This seemingly simple number holds the key to understanding oxygen's unique characteristics and its critical role in various biological and chemical processes.
Oxygen's Electron Configuration: [He] 2s²2p⁴
The eight electrons in an oxygen atom are distributed across two energy levels. Two electrons occupy the first energy level (1s²), while the remaining six electrons fill the second energy level (2s²2p⁴). This specific electron configuration is responsible for oxygen's high reactivity and its ability to form strong chemical bonds.
The presence of two unpaired electrons in the 2p orbitals makes oxygen highly reactive, readily accepting electrons to achieve a stable electron configuration, similar to that of noble gases. This drives its participation in numerous chemical reactions, some of which are essential for life.
Oxygen's Isotopes: Variations in Neutron Number
While the atomic number (number of protons) defines an element, the number of neutrons in the nucleus can vary. These variations are known as isotopes. Oxygen has three main stable isotopes:
-
Oxygen-16 (¹⁶O): This is the most abundant isotope, comprising about 99.76% of naturally occurring oxygen. It has 8 protons and 8 neutrons.
-
Oxygen-17 (¹⁷O): This isotope has 8 protons and 9 neutrons, making up approximately 0.04% of naturally occurring oxygen.
-
Oxygen-18 (¹⁸O): This isotope contains 8 protons and 10 neutrons, accounting for about 0.20% of naturally occurring oxygen.
Although these isotopes have different neutron numbers, their chemical properties remain largely unchanged because the number of protons (and therefore electrons) remains constant. However, the differences in mass can be exploited in various scientific applications, such as tracing water movement in hydrological studies or analyzing metabolic processes in organisms.
The Significance of Oxygen's Atomic Number in Biological Systems
Oxygen's atomic number of 8 directly impacts its critical role in sustaining life on Earth. Its high reactivity, driven by its electron configuration, enables it to participate in vital biological processes, including:
1. Cellular Respiration: The Energy Engine of Life
Oxygen serves as the final electron acceptor in cellular respiration, the process by which organisms convert energy from food into a usable form (ATP). The highly electronegative nature of oxygen (its strong attraction for electrons) makes it ideal for accepting electrons at the end of the electron transport chain, releasing energy that is then harnessed to produce ATP. Without oxygen as the final electron acceptor, the process of cellular respiration would grind to a halt, leading to a lack of energy for life functions.
2. Oxygen's Role in Oxidation-Reduction Reactions (Redox Reactions)
Oxygen's involvement in redox reactions is widespread. Oxidation is the loss of electrons, while reduction is the gain of electrons. Oxygen is a powerful oxidizing agent, readily accepting electrons from other molecules. This property underlies many crucial biological processes, including the breakdown of nutrients, the synthesis of molecules, and the detoxification of harmful substances.
3. Oxygen's Importance in Maintaining Structural Integrity
Oxygen is a key component of many biological molecules, including water (H₂O), carbohydrates, lipids, proteins, and nucleic acids. Its presence within these molecules contributes to their overall structure, function, and stability, supporting the integrity of living organisms.
Oxygen in Industrial and Technological Applications
Beyond its biological significance, oxygen's atomic number and resultant properties have led to a wide range of industrial and technological applications:
-
Welding and Cutting: Oxygen's high reactivity makes it essential for high-temperature processes like welding and cutting metals. The combination of oxygen and a fuel source creates an extremely hot flame, capable of melting and cutting through various materials.
-
Medicine: Oxygen therapy is crucial in treating various respiratory conditions and enhancing oxygen levels in patients with impaired respiratory function. It is also used in cryosurgery, where extremely cold oxygen is utilized to destroy abnormal tissues.
-
Water Treatment: Oxygen is used in water treatment to oxidize pollutants and improve water quality. This process is effective in removing organic contaminants and harmful bacteria.
-
Chemical Industry: Oxygen is a crucial reactant in numerous chemical processes, serving as an oxidizing agent in the production of various chemicals, including alcohols, acids, and other essential compounds.
Conclusion: The Significance of a Simple Number
The atomic number of oxygen, a seemingly simple number 8, holds immense significance. It dictates the element's chemical properties, its reactivity, and ultimately, its vital role in sustaining life and driving various industrial processes. Understanding this fundamental property is crucial for appreciating oxygen's central place in the universe and its influence on both the natural world and human civilization. The implications of this seemingly simple number extend far beyond the periodic table, influencing everything from the air we breathe to the technologies we rely upon. Further research into oxygen’s properties continues to reveal new aspects of its importance and its potential for future applications.
Latest Posts
Latest Posts
-
How Are Pressure And Temp Related
Mar 21, 2025
-
Is Burning Gasoline A Chemical Change
Mar 21, 2025
-
Which Is The Central Element For All Living Things
Mar 21, 2025
-
Acid And Base Extraction Lab Report
Mar 21, 2025
-
Where Are Prolines Found On An Alpha Helix
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is Atomic Number Of Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
