What Is End Point In Titration
Muz Play
Mar 31, 2025 · 5 min read
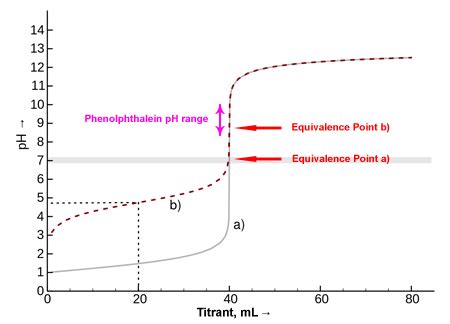
Table of Contents
What is the Endpoint in Titration? A Comprehensive Guide
Titration is a fundamental analytical technique used in chemistry to determine the concentration of an unknown solution (analyte) by reacting it with a solution of known concentration (titrant). Understanding the endpoint in titration is crucial for accurate and reliable results. This comprehensive guide delves into the intricacies of the endpoint, explaining its significance, different methods of detection, and potential sources of error.
Understanding the Basics of Titration
Before diving into the endpoint, let's revisit the core principles of titration. The process involves the gradual addition of the titrant to the analyte until the reaction between them is complete. This completion point is what we aim to identify, and it's signified by the endpoint. The reaction is typically an acid-base neutralization, a redox reaction, or a precipitation reaction, depending on the nature of the analyte and titrant.
Key Components of a Titration:
- Analyte: The solution of unknown concentration that we are trying to determine.
- Titrant: The solution of known concentration added to the analyte.
- Burette: A graduated glass tube used to deliver the titrant precisely.
- Erlenmeyer flask or beaker: Container holding the analyte.
- Indicator (optional): A substance that changes color near the equivalence point, helping to visually identify the endpoint.
What is the Endpoint in Titration?
The endpoint in titration is the point at which the indicator (if used) changes color, signaling the apparent completion of the reaction between the analyte and the titrant. It's the point at which we stop adding the titrant. Importantly, the endpoint isn't necessarily identical to the equivalence point.
Equivalence Point vs. Endpoint: A Crucial Distinction
The equivalence point is the theoretical point at which the moles of titrant added are stoichiometrically equivalent to the moles of analyte present. It represents the true completion of the chemical reaction. It's an invisible point, determined by calculations based on the stoichiometry of the reaction.
The difference between the equivalence point and the endpoint is called the endpoint error. This error arises because indicators don't always change color precisely at the equivalence point. The magnitude of the endpoint error depends on several factors, including the choice of indicator, the concentration of the solutions, and the temperature. Minimizing this error is essential for obtaining accurate results.
Methods of Endpoint Detection
Several methods are employed to detect the endpoint in titration, each with its advantages and limitations:
1. Visual Indicators:
Visual indicators are the most common method, relying on a color change. Acid-base titrations frequently use indicators like phenolphthalein (colorless in acidic solutions, pink in basic solutions) or methyl orange (red in acidic solutions, yellow in basic solutions). The choice of indicator is crucial; it should have a pKa close to the pH at the equivalence point to minimize the endpoint error. However, visual indicators can be subjective, relying on the observer's perception of color change.
2. Instrumental Methods:
Instrumental methods offer more precise and objective endpoint detection. These include:
-
pH meter: Continuously monitors the pH of the solution during titration, providing a precise plot of pH versus volume of titrant added. The equivalence point is identified as the point of maximum slope on the titration curve. This method is particularly useful for weak acid-weak base titrations where visual indicators are less effective.
-
Conductivity meter: Measures the electrical conductivity of the solution. The equivalence point is often marked by a sharp change in conductivity. This method is especially suitable for titrations involving ionic species.
-
Spectrophotometry: Monitors the absorbance of light by the solution at a specific wavelength. The absorbance changes significantly near the equivalence point. This method is widely used for redox titrations and other reactions where a significant change in absorbance occurs.
-
Potentiometry: Measures the potential difference between an indicator electrode and a reference electrode. The potential changes significantly near the equivalence point. This is often used in redox titrations and potentiometric titrations involving ion-selective electrodes.
Factors Affecting Endpoint Detection
Several factors can influence the accuracy of endpoint detection:
-
Indicator choice: Selecting an inappropriate indicator can lead to significant endpoint error. The indicator's pKa should be as close as possible to the pH at the equivalence point.
-
Solution concentration: Dilute solutions can make endpoint detection more challenging due to smaller color changes.
-
Temperature: Temperature variations can affect the equilibrium constant of the reaction and the indicator's color change.
-
Solution impurities: The presence of impurities can interfere with the reaction and lead to inaccurate endpoint determination.
-
Observer bias: In visual titrations, the observer's perception of color change can introduce subjectivity and error.
Minimizing Endpoint Error
To minimize endpoint error and improve the accuracy of titration results, consider these strategies:
-
Careful selection of the indicator: Choose an indicator with a pKa close to the expected pH at the equivalence point.
-
Using a suitable instrumental method: Instrumental methods provide more objective and precise endpoint detection.
-
Employing a blank titration: Performing a blank titration (titrating the titrant against a solvent without the analyte) can correct for any impurities in the titrant.
-
Maintaining consistent temperature: Keep the solution temperature relatively constant throughout the titration.
-
Using accurate measuring equipment: Ensure accurate measurements of both analyte and titrant volumes.
-
Appropriate mixing: Thorough mixing of the analyte and titrant is crucial to ensure complete reaction.
Applications of Titration and Endpoint Determination
Titration is a ubiquitous technique in various fields, including:
-
Environmental analysis: Determining the concentration of pollutants in water and air samples.
-
Food science: Analyzing the acidity of food products.
-
Pharmaceutical industry: Ensuring the purity and potency of drugs.
-
Clinical chemistry: Measuring the concentration of electrolytes and metabolites in biological fluids.
-
Industrial chemistry: Monitoring the quality of industrial chemicals and products.
Conclusion
The endpoint in titration is a critical aspect of this analytical technique. While it often serves as a practical approximation of the equivalence point, understanding the difference between the two, the various methods of detection, and the factors influencing endpoint determination are crucial for obtaining accurate and reliable results. By employing appropriate techniques and minimizing potential sources of error, titration can provide valuable information across numerous scientific and industrial applications. Mastering endpoint detection is key to accurate and reliable chemical analysis.
Latest Posts
Latest Posts
-
Is As A Metal Nonmetal Or Metalloid
Apr 02, 2025
-
The Process Of Getting Information Into Memory Is Called
Apr 02, 2025
-
Where Are Nonmetals Located In The Periodic Table
Apr 02, 2025
-
Write An Equation Any Form For The Quadratic Graphed Below
Apr 02, 2025
-
What Is The Chromosomal Basis Of Inheritance
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is End Point In Titration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
