What Is Oxygen's Number Of Protons
Muz Play
Mar 15, 2025 · 6 min read
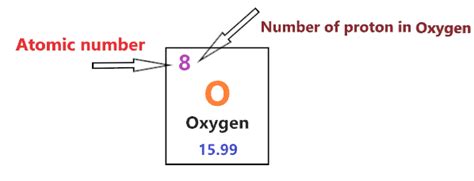
Table of Contents
What is Oxygen's Number of Protons? A Deep Dive into Atomic Structure
Oxygen, the life-sustaining gas we breathe, is more than just something we need to survive. It's a fascinating element with a unique atomic structure that dictates its properties and behavior. Understanding this structure, particularly the number of protons it possesses, is fundamental to grasping its role in chemistry and biology. This article will delve deep into the atomic structure of oxygen, exploring its protons, neutrons, electrons, and isotopes, as well as the implications of its atomic number in various scientific fields.
Understanding Atomic Structure: The Foundation of Oxygen's Properties
Before we pinpoint the number of protons in oxygen, let's establish a basic understanding of atomic structure. An atom is the fundamental building block of matter, consisting of a central nucleus surrounded by orbiting electrons. The nucleus itself is composed of two types of particles: protons and neutrons.
- Protons: Positively charged particles that contribute to an atom's atomic number and determine its identity as a specific element.
- Neutrons: Neutral particles (no charge) that contribute to an atom's mass but not its charge.
- Electrons: Negatively charged particles that orbit the nucleus in shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
The atomic number of an element is equal to the number of protons in its nucleus. This number is unique to each element and is fundamental in organizing the periodic table.
Oxygen's Atomic Number and the Number of Protons
Now, let's get to the heart of the matter: Oxygen's atomic number is 8. This means that every oxygen atom contains 8 protons in its nucleus. This fundamental fact is crucial because:
- It defines oxygen: The presence of 8 protons unequivocally identifies an atom as oxygen. Any atom with a different number of protons would be a different element.
- It determines its chemical properties: The number of protons dictates the number of electrons in a neutral oxygen atom. These electrons participate in chemical bonding, influencing oxygen's reactivity and how it interacts with other elements. Oxygen's strong electronegativity, its tendency to attract electrons, is directly related to its electron configuration, which is determined by its 8 protons.
- It helps understand its isotopes: Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. While the number of protons remains constant (8 for oxygen), the number of neutrons can vary, leading to different isotopes of oxygen.
Oxygen Isotopes: A Variation on a Theme
While the number of protons always remains 8 in an oxygen atom, the number of neutrons can vary, resulting in different isotopes. The most common isotopes of oxygen are:
- Oxygen-16 (¹⁶O): This is the most abundant isotope, comprising about 99.76% of naturally occurring oxygen. It contains 8 protons and 8 neutrons.
- Oxygen-17 (¹⁷O): This isotope is much rarer, making up about 0.04% of natural oxygen. It contains 8 protons and 9 neutrons.
- Oxygen-18 (¹⁸O): This isotope is also relatively rare, about 0.20% of natural oxygen. It contains 8 protons and 10 neutrons.
Despite the differences in neutron numbers, all three isotopes still have 8 protons, maintaining their identity as oxygen. The differing neutron numbers, however, lead to slight variations in mass and some subtle differences in their chemical behavior. These differences are often exploited in scientific techniques like isotopic tracing.
The Importance of Oxygen's Protons in Chemical Reactions
Oxygen's 8 protons are not just a matter of atomic bookkeeping; they play a crucial role in its chemical behavior. Oxygen's strong electronegativity, its tendency to attract electrons, stems directly from its electron configuration, which is dictated by the number of protons.
This electronegativity leads to the formation of numerous stable and important chemical bonds. Consider:
- Oxidation: Oxygen readily accepts electrons from other atoms, a process known as oxidation. This process is fundamental to many biological processes, including cellular respiration, where oxygen acts as the final electron acceptor in the electron transport chain, generating energy for the organism.
- Water Formation: Oxygen's ability to form covalent bonds with two hydrogen atoms leads to the formation of water (H₂O). The properties of water—its high surface tension, its ability to act as a solvent, and its specific heat—are directly related to the oxygen atom's electronegativity and the polar nature of the O-H bonds.
- Oxides: Oxygen reacts with many elements to form oxides. These compounds are ubiquitous in the environment and in industrial processes. The properties of oxides vary widely depending on the element involved, but oxygen's involvement is a common thread.
Oxygen's Role in Biology: Life's Essential Element
The significance of oxygen's 8 protons extends far beyond simple chemistry. Its crucial role in biology underscores the fundamental importance of its atomic structure. Oxygen is essential for aerobic respiration, the process by which organisms convert energy from food molecules. Without the presence of oxygen as the final electron acceptor, this process would not be efficient enough to sustain life as we know it.
Consider the following aspects of oxygen's biological importance:
- Cellular Respiration: The electron transport chain, a key stage in cellular respiration, relies on oxygen to accept electrons. This process generates a significant amount of ATP (adenosine triphosphate), the cell's primary energy currency.
- Oxygen Transport: Hemoglobin in red blood cells efficiently transports oxygen throughout the body, making it readily available for cellular respiration. The oxygen molecule's ability to bind to hemoglobin is due to its specific chemical properties, rooted in its atomic structure.
- Reactive Oxygen Species (ROS): While oxygen is essential for life, it can also participate in the formation of reactive oxygen species (ROS), such as superoxide radicals and hydrogen peroxide. These species can be damaging to cells if not properly controlled by antioxidant systems.
Oxygen in Various Scientific Fields: Applications and Research
The knowledge of oxygen's 8 protons and its atomic structure has profound implications in various scientific fields:
- Environmental Science: Monitoring oxygen levels in the atmosphere and oceans is crucial for understanding climate change and environmental health. Understanding the chemical behavior of oxygen allows scientists to model its interactions with other atmospheric components and assess its impact on the environment.
- Materials Science: Oxygen's reactivity is exploited in materials science to synthesize new materials with specific properties. For instance, the oxidation of metals can create protective layers against corrosion, while the inclusion of oxygen in certain ceramics alters their electrical or mechanical characteristics.
- Medical Science: Oxygen therapy is a vital treatment for various medical conditions, including respiratory distress. Understanding oxygen's behavior in the body is essential for developing effective treatments and interventions.
Conclusion: A Simple Number with Vast Implications
Oxygen's seemingly simple atomic number—8, representing 8 protons—underpins its fundamental chemical and biological properties. This number defines oxygen's identity as an element, dictates its chemical reactivity, and ultimately determines its irreplaceable role in supporting life on Earth. From the macroscopic level of atmospheric processes to the microscopic realm of cellular respiration, the significance of oxygen's eight protons extends far beyond its simple numerical value. Its study continues to drive advancements in numerous scientific disciplines and remains an area of ongoing research and exploration. The profound implications of this fundamental number highlight the power and beauty of the interconnectedness of atomic structure and the world around us.
Latest Posts
Latest Posts
-
Que Es La Descomposicion De Acidos
Mar 15, 2025
-
Which Factor Affects Congressional Approval Ratings The Most
Mar 15, 2025
-
Fourier Transform Of A Differential Equation
Mar 15, 2025
-
Which One Neutral Charge Proton Or Neutron
Mar 15, 2025
-
A Solids Volume And Shape Is Defintie
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is Oxygen's Number Of Protons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
