What Is The Difference Between A Solvent And Solute
Muz Play
Mar 20, 2025 · 6 min read
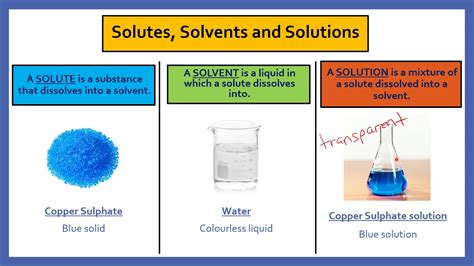
Table of Contents
What's the Difference Between a Solvent and a Solute? A Deep Dive into Solutions
Understanding the difference between a solvent and a solute is fundamental to grasping the nature of solutions, a concept crucial across various scientific disciplines, from chemistry and biology to environmental science and materials engineering. While seemingly simple, the nuances of solvent-solute interactions are surprisingly complex and hold the key to unlocking numerous chemical and physical phenomena. This comprehensive guide will delve into the definitions, properties, and behaviors of solvents and solutes, exploring their interactions and providing real-world examples to solidify your understanding.
Defining Solvents and Solutes: The Foundation of Solutions
At the heart of the matter lies the solution itself – a homogeneous mixture of two or more substances. This homogeneity is key; a solution appears uniform throughout, meaning its composition is consistent irrespective of the sample location. Within this homogenous mixture, we have two main components:
The Solvent: The Dissolver
The solvent is the substance that does the dissolving. It's typically the component present in the larger quantity and forms the continuous phase of the solution. Think of it as the medium in which the other component dissolves. The most common solvent is water, owing to its exceptional ability to dissolve a wide range of substances, earning it the title of "universal solvent." However, many other substances can act as solvents, depending on the solute and the conditions.
Key Characteristics of Solvents:
- High dissolving power: Solvents must possess the ability to attract and interact with solute particles, effectively breaking down the solute's structure.
- Suitable polarity: The polarity of the solvent plays a critical role. "Like dissolves like" is a fundamental principle: polar solvents generally dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes.
- Chemical stability: A good solvent should remain chemically stable and not react significantly with the solute.
- Appropriate boiling point: The boiling point should allow for easy separation of the solute after the process is complete.
The Solute: The Dissolved
The solute is the substance that gets dissolved. It's typically present in a smaller quantity than the solvent and is dispersed throughout the solvent at the molecular or ionic level. The solute’s properties are significantly altered when dissolved in the solvent.
Key Characteristics of Solutes:
- Dissolvability: The solute must possess the ability to be dissolved by the solvent. This depends on factors like polarity, molecular structure, and intermolecular forces.
- Concentration: The amount of solute dissolved in a given amount of solvent determines the concentration of the solution. This concentration can be expressed in various ways, including molarity, molality, and percentage by mass or volume.
- Solubility: Solubility refers to the maximum amount of solute that can be dissolved in a given amount of solvent at a specific temperature and pressure. Once this limit is reached, the solution becomes saturated.
The Interplay of Solvent and Solute: Understanding the Dissolution Process
The process of dissolution involves several complex steps. It’s not merely a case of one substance disappearing into another. Instead, it’s a dynamic interaction governed by intermolecular forces:
-
Solvent-Solute Interaction: The solvent molecules approach the solute particles. If the solvent is polar and the solute is polar (or ionic), the solvent molecules' partially charged regions attract the solute particles' oppositely charged regions. This attraction weakens the forces holding the solute particles together. For nonpolar solutes and solvents, London dispersion forces are the primary interaction.
-
Breaking of Intermolecular Forces: The attractive forces between solute particles (e.g., hydrogen bonds, dipole-dipole interactions, ionic bonds) are overcome by the attractive forces between solvent and solute particles. This requires energy input.
-
Solvation (or Hydration): Once separated, the solute particles become surrounded by solvent molecules. This process is called solvation. If the solvent is water, it’s specifically termed hydration. The solute particles are now effectively isolated from each other and dispersed throughout the solvent.
-
Equilibrium: The process continues until a dynamic equilibrium is reached. At this point, the rate of solute dissolving equals the rate of solute recrystallization (solute particles coming back together). A saturated solution represents this equilibrium state.
Factors Affecting Solubility: Temperature, Pressure, and More
Several factors significantly influence the solubility of a solute in a given solvent:
-
Temperature: For most solid solutes, solubility increases with increasing temperature. The added energy helps overcome the intermolecular forces holding the solute together. However, for gaseous solutes, solubility generally decreases with increasing temperature. Increased kinetic energy allows gas molecules to escape the solution more readily.
-
Pressure: Pressure has a significant effect on the solubility of gases. According to Henry's Law, the solubility of a gas is directly proportional to the partial pressure of that gas above the solution. Increased pressure forces more gas molecules into the solution. Pressure has minimal effect on the solubility of solids and liquids.
-
Nature of the Solvent and Solute: As mentioned earlier, "like dissolves like" is a crucial principle. Polar solvents effectively dissolve polar and ionic solutes, while nonpolar solvents dissolve nonpolar solutes. The strength and type of intermolecular forces play a significant role in determining solubility.
-
Presence of Other Substances: The presence of other substances in the solution can affect solubility. For example, the common-ion effect reduces the solubility of a sparingly soluble salt when a common ion is added to the solution.
Types of Solutions: Exploring Different Scenarios
Solutions are not a one-size-fits-all concept. Several factors influence their properties, leading to different types of solutions:
-
Aqueous Solutions: These solutions have water as the solvent. Aqueous solutions are ubiquitous in biological systems and many chemical processes.
-
Non-Aqueous Solutions: These solutions employ solvents other than water, such as ethanol, acetone, benzene, or hexane. The choice of solvent is crucial for dissolving specific solutes that are insoluble in water.
-
Saturated Solutions: A saturated solution contains the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure. Any additional solute will simply remain undissolved.
-
Unsaturated Solutions: An unsaturated solution contains less solute than the maximum solubility allows. More solute can be dissolved in an unsaturated solution.
-
Supersaturated Solutions: A supersaturated solution contains more solute than its maximum solubility allows. These solutions are unstable and tend to precipitate out excess solute if disturbed.
Real-World Applications: The Importance of Solvents and Solutes
Understanding the interplay between solvents and solutes has profound implications across various fields:
-
Medicine: Many pharmaceuticals are administered as solutions. The choice of solvent is critical for drug delivery, bioavailability, and minimizing adverse reactions.
-
Biology: Biological systems are largely aqueous solutions. The dissolution and interaction of various molecules in water are essential for cellular processes, enzymatic activity, and metabolic pathways.
-
Environmental Science: Understanding the solubility of pollutants is crucial for assessing their environmental impact and developing remediation strategies. Solubility determines how pollutants move through the environment and affect ecosystems.
-
Chemistry and Materials Science: Solvents are used extensively in chemical synthesis, extraction processes, and the preparation of various materials. Controlling the solvent and solute properties is crucial for optimizing reaction yields and material properties.
Conclusion: A Deeper Understanding of Solutions
The distinction between solvents and solutes, while seemingly straightforward, opens the door to a fascinating world of chemical interactions and physical processes. Understanding their properties, interactions, and the factors influencing their behavior is essential for anyone working in fields involving solutions, from basic research to advanced applications in medicine, engineering, and environmental science. The more we delve into the intricate dance between solvents and solutes, the more we uncover the secrets hidden within the seemingly simple concept of a solution. This exploration emphasizes the crucial role that understanding fundamental concepts plays in unlocking the complexities of the world around us.
Latest Posts
Latest Posts
-
Humidity Is Measured With What Instrument
Mar 20, 2025
-
The Elite Theory Of Government Maintains That
Mar 20, 2025
-
What Elements Have An Expanded Octet
Mar 20, 2025
-
Fundamentals Of General Organic And Biological Chemistry
Mar 20, 2025
-
How To Calculate Kb From Ka
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between A Solvent And Solute . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
