What Is The Difference Between A Weak And Strong Acid
Muz Play
Mar 21, 2025 · 6 min read
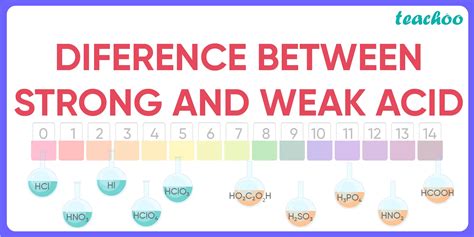
Table of Contents
What's the Difference Between a Weak and a Strong Acid? A Deep Dive into Acid Strength
Acids are ubiquitous in our lives, from the citric acid in oranges to the sulfuric acid used in car batteries. Understanding the difference between weak and strong acids is crucial for anyone studying chemistry, or simply curious about the world around them. This article delves deep into the distinctions, exploring the concepts of dissociation, pH, Ka, and pKa, and providing real-world examples to solidify your understanding.
The Fundamental Difference: Dissociation in Water
The core difference between a strong acid and a weak acid lies in their degree of dissociation in water. Dissociation is the process where an acid molecule (HA) donates a proton (H⁺) to a water molecule, forming a hydronium ion (H₃O⁺) and an anion (A⁻).
Strong acids completely dissociate in water. This means that virtually every molecule of the acid donates its proton. The equilibrium lies heavily on the product side of the reaction:
HA(aq) + H₂O(l) → H₃O⁺(aq) + A⁻(aq)
Weak acids, on the other hand, only partially dissociate. A significant portion of the acid molecules remain undissociated in solution. The equilibrium lies significantly towards the reactants:
HA(aq) + H₂O(l) ⇌ H₃O⁺(aq) + A⁻(aq)
Notice the use of a double arrow (⇌) for weak acids, indicating a reversible reaction, compared to a single arrow (→) for strong acids. This reversible nature is key to understanding the behavior of weak acids.
pH: A Measure of Acidity
The pH scale is a logarithmic scale that measures the concentration of hydronium ions (H₃O⁺) in a solution. A lower pH indicates a higher concentration of H₃O⁺ and therefore a stronger acidic solution.
-
Strong acids have a significantly lower pH than weak acids at the same concentration. This is because they produce a much higher concentration of H₃O⁺ ions upon dissociation.
-
Weak acids have a higher pH than strong acids at the same concentration, reflecting their incomplete dissociation and lower H₃O⁺ concentration.
Ka and pKa: Quantifying Acid Strength
While pH provides a measure of acidity in a particular solution, the acid dissociation constant (Ka) provides a more fundamental measure of acid strength, independent of concentration. Ka is the equilibrium constant for the dissociation reaction:
Ka = [H₃O⁺][A⁻] / [HA]
A higher Ka value indicates a stronger acid because it means that a greater proportion of the acid has dissociated into ions at equilibrium.
Because Ka values can span a vast range, the pKa value is often used instead. pKa is the negative logarithm (base 10) of Ka:
pKa = -log₁₀(Ka)
A lower pKa value indicates a stronger acid. A difference of one pKa unit represents a tenfold difference in acid strength.
Common Strong Acids and Their Characteristics
The common strong acids are easily remembered using the mnemonic "HBr, HCl, HI, HNO₃, H₂SO₄, HClO₄". These acids completely dissociate in aqueous solutions:
- Hydrochloric acid (HCl): Found in stomach acid, used in industrial cleaning.
- Hydrobromic acid (HBr): Used in the production of certain organic compounds.
- Hydroiodic acid (HI): Also used in organic synthesis.
- Nitric acid (HNO₃): Used in fertilizer production and explosives manufacturing.
- Sulfuric acid (H₂SO₄): Widely used in industry, including fertilizer production, oil refining, and battery manufacturing. Note that it's a diprotic acid, meaning it can donate two protons. The first dissociation is essentially complete, but the second is not.
- Perchloric acid (HClO₄): One of the strongest known acids, often used in analytical chemistry.
These strong acids demonstrate complete dissociation, leading to high concentrations of H₃O⁺ ions and low pH values in solution.
Common Weak Acids and Their Applications
Weak acids are much more prevalent than strong acids. Examples include:
- Acetic acid (CH₃COOH): The main component of vinegar, used in food preservation and as a cleaning agent.
- Citric acid: Found in citrus fruits, used as a flavoring agent and preservative.
- Carbonic acid (H₂CO₃): Formed when carbon dioxide dissolves in water, plays a crucial role in regulating blood pH.
- Formic acid (HCOOH): Found in ant stings and bee venom.
- Benzoic acid (C₆H₅COOH): Used as a food preservative and in the production of some plastics.
- Phosphoric acid (H₃PO₄): Used in fertilizers and detergents, also found in some soft drinks. Like sulfuric acid, it's polyprotic.
These weak acids exhibit only partial dissociation, resulting in lower H₃O⁺ concentrations and higher pH values compared to strong acids at the same concentration. Their behavior is governed by their respective Ka and pKa values.
Factors Affecting Acid Strength
Several factors influence the strength of an acid:
- Bond strength: The strength of the bond between the hydrogen atom and the rest of the molecule plays a significant role. Weaker bonds lead to easier proton donation and stronger acidity. For example, the H-I bond is weaker than the H-Cl bond, resulting in HI being a stronger acid than HCl.
- Electronegativity: The electronegativity of the atom bonded to the hydrogen atom affects the polarity of the bond. Higher electronegativity leads to a more polarized bond, making it easier to donate the proton.
- Size and stability of the conjugate base: The conjugate base is the anion (A⁻) formed after the acid donates its proton. A more stable conjugate base makes the acid stronger. Stability is often enhanced by resonance effects or inductive effects.
- Solvent effects: The solvent in which the acid is dissolved can also affect its strength.
Practical Applications of Understanding Acid Strength
The difference between weak and strong acids has significant implications in various applications:
- Industrial processes: The choice of acid in industrial processes depends on its strength and reactivity. Strong acids are needed for reactions requiring complete dissociation, while weak acids are preferred in situations where milder conditions are necessary.
- Medicine and pharmaceuticals: The strength of acids affects their bioavailability and interaction with biological systems. Weak acids are often used in pharmaceuticals due to their controlled dissociation.
- Environmental science: The acidity of rainwater and other environmental samples is crucial for assessing environmental quality and potential impacts. Understanding acid strength is necessary for interpreting these measurements.
- Food science: The acidity of food products influences their flavor, preservation, and safety. Weak acids are commonly used as food preservatives.
Conclusion: A Crucial Distinction
The distinction between weak and strong acids is fundamental in chemistry. While both donate protons, their degree of dissociation significantly impacts their properties and applications. Understanding the concepts of dissociation, pH, Ka, and pKa, as well as the factors influencing acid strength, is crucial for comprehending a wide range of chemical and biological phenomena. This knowledge is essential in diverse fields, from industrial processes to environmental monitoring and medical applications. The exploration of weak and strong acids offers a fascinating glimpse into the complexity and versatility of chemical reactions. By appreciating the nuances of acid strength, we can gain a deeper understanding of the chemical world around us.
Latest Posts
Latest Posts
-
Nuclear Power Plant Steam Heat Temprature
Mar 22, 2025
-
Periodic Table Color Coded Metals Nonmetals Metalloids
Mar 22, 2025
-
Which Step Of Cellular Respiration Produces The Most Atp
Mar 22, 2025
-
How Do You Calculate Ph From Molarity
Mar 22, 2025
-
What Is The Electron Configuration For Lithium
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between A Weak And Strong Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
