Periodic Table Color Coded Metals Nonmetals Metalloids
Muz Play
Mar 22, 2025 · 7 min read
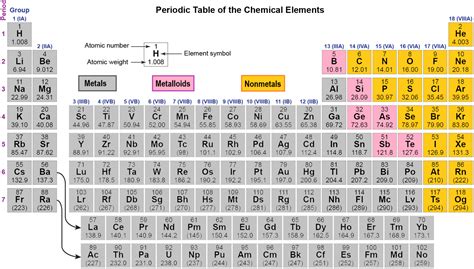
Table of Contents
Decoding the Periodic Table: A Colorful Guide to Metals, Nonmetals, and Metalloids
The periodic table, that iconic chart adorning countless science classrooms, is more than just a list of elements. It's a visual representation of the fundamental building blocks of matter, organized by their properties and atomic structure. One of the most striking features of many periodic tables is the color-coding, a system that helps us quickly identify elements as metals, nonmetals, or metalloids – categories based on their shared physical and chemical characteristics. This article will delve deep into the colorful world of the periodic table, explaining the distinctions between these three groups and exploring their unique properties.
Understanding the Color-Coded System
Before diving into the specifics of each category, let's establish the basic color-coding convention. While slight variations exist across different periodic tables, the general scheme is fairly consistent:
-
Metals: Typically represented by shades of blue, silver, or other metallic colors. The color choice reflects their characteristic shiny, lustrous appearance.
-
Nonmetals: Often depicted in various shades of yellow, orange, green, or even pink. This diverse palette reflects the broader range of physical properties found within this group.
-
Metalloids (Semimetals): Usually shown in shades of purple, pink, or light green. This distinct color highlights their intermediate nature, possessing properties of both metals and nonmetals.
The Metallic Realm: A Sea of Shared Characteristics
Metals, the dominant players on the periodic table, occupy the left and center sections. Their abundance and widespread use in everyday life are testaments to their remarkable properties. Several key characteristics define this vast group:
1. Conductivity: The Flow of Electricity and Heat
Perhaps the most defining characteristic of metals is their exceptional conductivity. Metals are excellent conductors of both electricity and heat. This property stems from the unique arrangement of their electrons, which are loosely bound to their atoms and can move freely throughout the metallic structure. This free movement of electrons allows for the efficient transfer of electrical charge and thermal energy. This is why metals are extensively used in electrical wiring and cookware.
2. Malleability and Ductility: Shaping Metals with Ease
Metals exhibit impressive malleability, meaning they can be easily hammered or pressed into different shapes without breaking. They are also ductile, capable of being drawn into thin wires. These properties are due to the ability of metal atoms to slide past one another without disrupting the overall metallic bonding. This makes them highly desirable for crafting intricate objects and components.
3. Luster: The Shimmering Surface
The lustrous, shiny appearance of metals is another hallmark characteristic. This metallic sheen results from the interaction of light with the free electrons in the metal's structure. These electrons absorb and re-emit light across a wide range of wavelengths, creating the characteristic metallic gleam.
4. Density and Hardness: A Range of Physical Properties
While not universally true across all metals, many possess relatively high density and hardness compared to nonmetals. This is linked to the strong metallic bonds that hold their atoms tightly together. However, there is significant variation within the metallic group; some metals are exceptionally dense (like osmium), while others are relatively lightweight (like lithium). Similarly, hardness varies significantly across different metals.
The Nonmetal Neighborhood: A Diverse Group
Nonmetals are located on the upper right-hand side of the periodic table. Unlike metals, they generally lack the characteristic properties of conductivity, malleability, and ductility. Instead, they exhibit a more diverse range of properties:
1. Poor Conductors: Resisting the Flow
Nonmetals are generally poor conductors of both electricity and heat. This is because their electrons are tightly bound to their atoms, limiting their ability to move freely and carry charge or energy. This property is utilized in applications where insulation is needed.
2. Brittle and Non-Ductile: Fragile Structures
Nonmetals tend to be brittle and lack ductility. They are prone to shattering under stress rather than deforming. This is a direct consequence of the strong covalent bonds that hold their atoms together in rigid, non-flexible structures.
3. Variable States: Gases, Liquids, and Solids
Nonmetals exist in all three states of matter at standard temperature and pressure. Several are gases (e.g., oxygen, nitrogen), one is a liquid (bromine), and others are solids (e.g., carbon, sulfur). This diversity in physical states reflects the wide variation in their interatomic forces.
4. Diverse Chemical Properties: Reactive and Unreactive
Nonmetals exhibit a broader range of chemical behaviors compared to metals. Some are highly reactive, readily forming compounds with other elements (e.g., oxygen, chlorine), while others are relatively inert (e.g., noble gases). This reactivity is crucial for understanding their roles in biological and chemical processes.
The Metalloid Middle Ground: A Blend of Properties
Metalloids, also known as semimetals, occupy a fascinating position between metals and nonmetals on the periodic table. They form a zig-zag line separating the two major groups. This intermediate location reflects their unique blend of metallic and nonmetallic properties:
1. Semiconductors: Controlling the Flow of Electricity
Metalloids are known for their semiconducting properties. This means they can conduct electricity under certain conditions, such as increased temperature or the addition of impurities (doping). This controlled conductivity makes them indispensable in electronic devices, forming the basis of transistors and integrated circuits.
2. Variable Physical Properties: A Mixture of Characteristics
Metalloids exhibit a mixture of metallic and nonmetallic physical properties. For instance, some may have a metallic luster but be brittle like nonmetals. This duality makes their categorization challenging, highlighting their unique position within the periodic table.
3. Important Applications: From Electronics to Medicine
The unique properties of metalloids make them crucial in a wide array of applications. Silicon, for example, is the backbone of the modern electronics industry. Boron is used in various alloys and glass manufacturing. Arsenic, despite its toxicity, has found use in certain medical treatments.
Exploring Specific Examples: A Closer Look
Let's examine a few key elements from each category to further illustrate their distinct characteristics:
Metals:
-
Iron (Fe): A strong, durable metal used extensively in construction, manufacturing, and transportation. Its excellent conductivity and malleability make it a versatile material.
-
Copper (Cu): A highly conductive metal, essential for electrical wiring and plumbing. Its reddish-brown color and resistance to corrosion contribute to its widespread use.
-
Gold (Au): A highly prized noble metal known for its inertness, malleability, and lustrous appearance. It is widely used in jewelry, electronics, and investments.
Nonmetals:
-
Oxygen (O₂): An essential gas for respiration, crucial for life on Earth. Its high reactivity enables it to form many important compounds.
-
Chlorine (Cl₂): A highly reactive gas used in water purification and the production of various chemicals. It's a powerful disinfectant but requires careful handling due to its toxicity.
-
Carbon (C): A versatile element existing in various allotropes, including diamond and graphite. It's the fundamental building block of organic molecules and is essential for life.
Metalloids:
-
Silicon (Si): The primary component of most semiconductors. Its unique electrical properties underpin the electronics revolution.
-
Arsenic (As): A toxic metalloid with limited applications, mainly used in specialized alloys and certain medications.
-
Germanium (Ge): Another semiconductor used in transistors and other electronic components. Its high refractive index makes it useful in optical fibers.
The Periodic Table: A Dynamic and Evolving Resource
The periodic table is not a static entity; our understanding of its elements is constantly evolving. New discoveries and advancements in technology continue to refine our knowledge of elemental properties and their applications. The color-coding system, while providing a helpful visual aid, is a simplification. Elements within each group exhibit a range of properties, and the lines between categories can sometimes be blurry. However, this color-coding remains a powerful tool for understanding the fundamental differences between metals, nonmetals, and metalloids, offering a crucial first step in exploring the fascinating world of chemistry.
Latest Posts
Latest Posts
-
Muscle Cells Use Lactic Acid Fermentation To
Mar 22, 2025
-
What Is The Principle Of Original Horizontality
Mar 22, 2025
-
What Are The Rows In The Periodic Table Called
Mar 22, 2025
-
How Many Valence Electrons Are In Neon
Mar 22, 2025
-
Iron Rust Chemical Or Physical Change
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Periodic Table Color Coded Metals Nonmetals Metalloids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
