What Are The Rows In The Periodic Table Called
Muz Play
Mar 22, 2025 · 6 min read
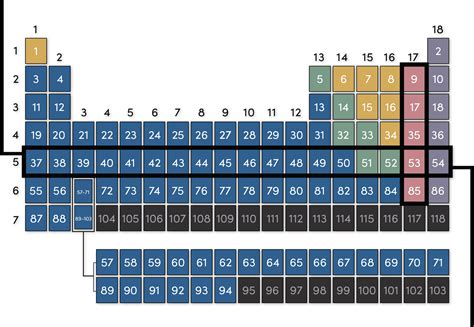
Table of Contents
What are the Rows in the Periodic Table Called? A Deep Dive into Periods and their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While most are familiar with the columns, known as groups or families, the horizontal rows also hold significant meaning and are known as periods. Understanding periods is crucial for grasping the trends and patterns in element behavior. This article delves deep into what periods are, why they're important, and how they relate to atomic structure and properties.
Understanding Periods: A Horizontal Journey Across the Elements
The rows of the periodic table, the periods, represent elements that share the same highest occupied principal energy level (or shell). This means that the electrons in the outermost shell of elements within the same period are at the same energy level. This seemingly simple fact underpins a wealth of predictable chemical and physical properties.
Period 1: The Simplest Beginnings
Period 1, the shortest period, contains only two elements: hydrogen (H) and helium (He). These elements have electrons only in the first principal energy level (n=1), which can hold a maximum of two electrons. This makes them fundamentally different from elements in subsequent periods.
Period 2 and 3: Building Complexity
Periods 2 and 3 each contain eight elements. In period 2, electrons begin to fill the second principal energy level (n=2), which includes the s and p subshells. This leads to a greater diversity of properties compared to Period 1. Similarly, period 3 sees the filling of the third principal energy level (n=3), again with s and p subshells. The increased number of electrons and the subsequent expansion of the electron cloud influence the elements' atomic radii, electronegativity, and ionization energies.
Periods 4 and 5: Transition Metals Emerge
Periods 4 and 5 are significantly longer than the previous periods due to the introduction of the d-block elements, also known as transition metals. These elements are characterized by the filling of the d subshells. The presence of these inner electrons influences the properties of transition metals, leading to variable oxidation states, complex ion formation, and catalytic activity. The increased number of electrons also affects their atomic size and other properties.
Periods 6 and 7: The Lanthanides, Actinides, and Beyond
Periods 6 and 7 are the longest periods, further expanding upon the complexities introduced in periods 4 and 5. The addition of the f-block elements—the lanthanides (period 6) and actinides (period 7)—significantly increases the number of elements within these periods. The f subshells are filled in these elements, impacting their chemical behavior and leading to a high degree of similarity in properties within the lanthanide and actinide series. Many of these elements are radioactive and exhibit unique properties.
The Significance of Periodicity: Trends and Patterns Across the Table
The arrangement of elements into periods is not arbitrary. It reflects a fundamental periodicity in their properties—recurring trends that can be predicted based on an element's position in the periodic table. These trends are directly linked to the filling of electron shells and subshells.
Atomic Radius: The Size Matters
Moving across a period from left to right, the atomic radius generally decreases. This is because the number of protons in the nucleus increases, leading to a stronger attraction for the electrons. This stronger pull effectively shrinks the electron cloud. However, there are exceptions to this trend, particularly in the transition metal series, due to electron shielding and electron-electron repulsion.
Ionization Energy: The Energy to Remove an Electron
Ionization energy is the energy required to remove an electron from an atom. As we move across a period, ionization energy generally increases. This is because the increasing nuclear charge holds the electrons more tightly. Removing an electron from an atom becomes progressively more difficult. Again, exceptions can arise due to the complexities of electron configurations in transition metals and other elements.
Electronegativity: The Battle for Electrons
Electronegativity refers to an atom's ability to attract electrons in a chemical bond. Electronegativity generally increases across a period. Elements on the right side of the table, such as halogens, are highly electronegative, meaning they strongly attract electrons during bonding. This is because they are closer to achieving a stable electron configuration.
Electron Affinity: Welcoming New Electrons
Electron affinity measures the energy change when an atom gains an electron. While there are variations, there's a general trend of increasing electron affinity across a period, as the stronger nuclear charge attracts the added electron. However, the trend is not as consistent as other periodic properties, and exceptions arise.
Periods and Chemical Properties: Predicting Reactivity
The periodic arrangement highlights how the number of valence electrons (electrons in the outermost shell) strongly influences the chemical behavior of an element. Elements within the same period share the same number of electron shells but have a different number of valence electrons. This difference in valence electron count accounts for the considerable variation in their chemical behavior.
Alkali Metals (Group 1): Highly Reactive
Alkali metals, located in the first column (group 1) of each period (except Period 1), have only one valence electron. This makes them highly reactive, readily losing this electron to form +1 ions and achieving a stable electron configuration. Their reactivity generally increases down the group.
Alkaline Earth Metals (Group 2): Reactive but Less so
Alkaline earth metals, in group 2, have two valence electrons. They are also reactive metals, losing two electrons to form +2 ions. Their reactivity is lower compared to alkali metals due to the stronger hold of the nucleus on the two electrons.
Halogens (Group 17): Electron-Hungry
Halogens, located in group 17, are highly reactive nonmetals with seven valence electrons. They readily gain one electron to form -1 ions, completing their outermost shell and achieving a stable noble gas configuration. Their reactivity generally decreases down the group.
Noble Gases (Group 18): The Inert Ones
Noble gases, in group 18, are unreactive due to their complete outermost electron shells. They have eight valence electrons (except helium, which has two), fulfilling the octet rule, making them chemically inert under normal conditions.
Periods and Physical Properties: Exploring Observable Trends
Periodicity also manifests in observable physical properties:
-
Melting and Boiling Points: These properties generally show a trend across a period. For example, in period 3, the melting and boiling points increase from sodium (Na) to silicon (Si) and then decrease, due to the changing nature of bonding.
-
Density: Density also demonstrates a trend, with elements in the middle of a period often showing higher densities than those at the ends. This correlates with the structure and bonding in the elements.
Conclusion: The Unfolding Story of Periods
The rows of the periodic table, the periods, are far more than just a horizontal arrangement of elements. They represent a fundamental organization of elements based on their electron configurations, driving the trends in their properties and chemical behavior. Understanding periods is essential for grasping the patterns and predictability in the world of chemistry. By understanding the significance of electron shells and their filling, we can better predict the properties of elements and explain their reactivity and interactions. The periodic table, with its periods and groups, provides a powerful and elegant framework for comprehending the diversity and interconnectedness of all the elements that form the basis of matter.
Latest Posts
Latest Posts
-
Cell A Basic Unit Of Life
Mar 22, 2025
-
What Is The Electron Configuration Of N
Mar 22, 2025
-
First Order Reaction Vs Second Order Reaction
Mar 22, 2025
-
Peritrichous Bacteria Make A Run When
Mar 22, 2025
-
Why Is Fractional Distillation Better Than Simple
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Are The Rows In The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
