What Is The Electron Configuration Of N
Muz Play
Mar 22, 2025 · 6 min read
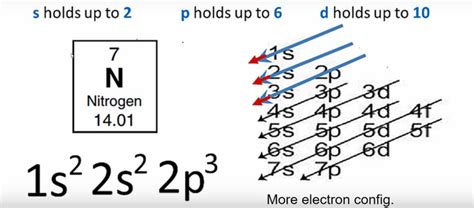
Table of Contents
What is the Electron Configuration of Nitrogen? Understanding Atomic Structure and its Implications
Nitrogen, a crucial element for life as we know it, boasts a fascinating electron configuration that dictates its chemical behavior and properties. Understanding this configuration is key to comprehending its role in everything from the air we breathe to the complex molecules that make up our DNA. This in-depth article will explore the electron configuration of nitrogen, its implications for chemical bonding, and its significance in various scientific fields.
Defining Electron Configuration
Before diving into nitrogen's specifics, let's establish a foundational understanding of electron configuration. It's a shorthand notation describing the arrangement of electrons within an atom's electron shells and subshells. These shells and subshells represent different energy levels, with electrons occupying the lowest energy levels first, following the Aufbau principle. This principle, coupled with the Pauli exclusion principle (which dictates that no two electrons can have the same quantum numbers) and Hund's rule (which favors maximizing unpaired electrons in degenerate orbitals), governs the precise electron configuration of any atom.
Electron configurations are typically represented using a series of numbers and letters. The numbers indicate the principal energy level (shell), while the letters (s, p, d, f) denote the subshells within those energy levels. Each letter is followed by a superscript indicating the number of electrons occupying that subshell.
Determining the Electron Configuration of Nitrogen (N)
Nitrogen (N) has an atomic number of 7, meaning it possesses 7 protons and 7 electrons in its neutral state. To determine its electron configuration, we systematically fill the electron shells according to the Aufbau principle:
-
First shell (n=1): This shell contains only the 1s subshell, which can accommodate a maximum of two electrons. Therefore, nitrogen's first shell is completely filled with two electrons: 1s².
-
Second shell (n=2): This shell consists of the 2s and 2p subshells. The 2s subshell, like the 1s, can hold a maximum of two electrons. So, we add two more electrons to nitrogen's configuration: 2s².
-
Second shell (n=2) continued: 2p subshell: The 2p subshell is slightly higher in energy than the 2s and can accommodate up to six electrons. However, nitrogen only has three more electrons to fill its orbitals. These three electrons occupy the three separate 2p orbitals individually before pairing up, in accordance with Hund's rule, leading to the configuration: 2p³.
Therefore, the complete electron configuration of nitrogen is: 1s²2s²2p³.
Visualizing Nitrogen's Electron Configuration
To gain a clearer picture, let's visualize the electron configuration using orbital diagrams. These diagrams provide a spatial representation of electron distribution within the atom's orbitals.
-
1s²: This indicates two electrons occupying the 1s orbital, represented as: ↑↓ (1s)
-
2s²: Two electrons fill the 2s orbital: ↑↓ (2s)
-
2p³: Three electrons occupy the three 2p orbitals individually, before pairing up (Hund's rule): ↑ (2px) ↑ (2py) ↑ (2pz)
Combining these, the full orbital diagram for nitrogen is: ↑↓ (1s) ↑↓ (2s) ↑ (2px) ↑ (2py) ↑ (2pz)
Implications of Nitrogen's Electron Configuration for Chemical Bonding
Nitrogen's electron configuration has profound implications for its chemical behavior, primarily its ability to form covalent bonds. The three unpaired electrons in the 2p subshell allow nitrogen to readily form three covalent bonds with other atoms. This is why nitrogen is commonly found in compounds such as ammonia (NH₃), where it forms three single bonds with three hydrogen atoms. Other examples of nitrogen's bonding capability include nitrogen trifluoride (NF₃) and numerous organic compounds containing nitrogen-carbon bonds.
Moreover, the presence of three unpaired electrons allows nitrogen to participate in multiple bonding. For instance, in dinitrogen (N₂), each nitrogen atom shares three electrons with the other, forming a triple bond (N≡N). This triple bond is exceptionally strong, contributing to the inertness and relative stability of diatomic nitrogen gas (N₂), the major component of Earth's atmosphere. This remarkable strength significantly impacts the atmospheric chemistry of our planet.
Nitrogen's Significance Across Scientific Disciplines
Nitrogen's unique electron configuration and consequent chemical properties underpin its significance in various scientific fields:
-
Biology: Nitrogen is a fundamental building block of proteins, nucleic acids (DNA and RNA), and amino acids. It's an essential nutrient for all living organisms, driving plant growth and forming crucial components in animal cells.
-
Chemistry: Its diverse bonding capabilities make nitrogen a key player in countless chemical reactions and synthetic processes. Nitrogen-containing compounds find applications in fertilizers, explosives, pharmaceuticals, and various industrial chemicals.
-
Atmospheric Science: Diatomic nitrogen (N₂) constitutes about 78% of the Earth's atmosphere. While relatively inert, its role in atmospheric processes and its interactions with other atmospheric components are crucial in understanding climate change and air quality.
-
Materials Science: Nitrogen plays a role in materials synthesis, modifying properties such as hardness, strength and corrosion resistance. Nitrogen doping is utilized to alter semiconductor properties.
-
Medicine: Nitrogen-based compounds have extensive applications in medical diagnosis and treatment. Nitric oxide (NO), for example, plays a role as a signaling molecule in the cardiovascular system. Nitrogen is a crucial component in several pharmaceuticals.
Advanced Concepts and Extensions
The simplistic explanation above provides a foundational understanding. More advanced concepts, however, can provide a deeper insight into the nuances of nitrogen's electronic structure:
-
Electron shielding: Inner electrons shield the outer electrons from the full nuclear charge, affecting the energy levels of outer electrons and subsequently influencing chemical behavior.
-
Effective Nuclear Charge: This represents the net positive charge experienced by outer electrons, considering the shielding effect of inner electrons. Understanding effective nuclear charge helps predict atomic radii and ionization energies.
-
Hybridization: In molecular orbitals, the atomic orbitals hybridize to form new orbitals that are more stable and contribute to molecular geometry. Nitrogen's sp³ hybridization in ammonia (NH₃) is a prime example.
-
Molecular Orbital Theory: A more complex method that offers a deeper understanding of chemical bonding, describing molecular orbitals as linear combinations of atomic orbitals. This theory offers insights into bond orders and magnetic properties.
Conclusion
The electron configuration of nitrogen, 1s²2s²2p³, is not merely a collection of numbers and letters. It's a concise code revealing the atom's fundamental structure and dictating its interactions with other atoms. This configuration explains nitrogen's ability to form stable covalent bonds, driving its central role in biological systems, chemical reactions, and atmospheric processes. From the air we breathe to the intricate molecules of life, nitrogen's unique electronic structure profoundly impacts our world. Further exploration into advanced concepts like electron shielding, effective nuclear charge, and molecular orbital theory provides a richer and more comprehensive understanding of this fundamental element's behavior and significance. As scientific research continues, our understanding of nitrogen's role in various disciplines will undoubtedly expand, emphasizing the fundamental importance of its seemingly simple electron configuration.
Latest Posts
Latest Posts
-
5 4 Practice Analyzing Graphs Of Polynomial Functions
Mar 23, 2025
-
Why Do Ionic Compounds Have High Melting Point
Mar 23, 2025
-
Are Shield Volcanoes Mafic Or Felsic
Mar 23, 2025
-
Does Sulfur Follow The Octet Rule
Mar 23, 2025
-
Temperature And Kinetic Energy Have A Relationship
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of N . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
