Why Is Fractional Distillation Better Than Simple
Muz Play
Mar 22, 2025 · 6 min read
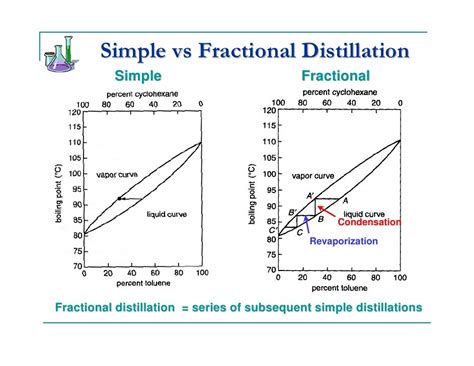
Table of Contents
Why Fractional Distillation Reigns Supreme: A Deep Dive into its Advantages over Simple Distillation
Distillation, the process of separating components or substances from a liquid mixture by using selective boiling and condensation, is a cornerstone technique in chemistry and various industries. While simple distillation serves its purpose in specific scenarios, fractional distillation emerges as the superior method for separating mixtures with boiling points that are relatively close together. This article will delve into the intricacies of both techniques, highlighting why fractional distillation significantly outperforms simple distillation in achieving efficient and high-purity separations.
Understanding Simple Distillation: Its Limitations and Applications
Simple distillation is a straightforward process involving heating a liquid mixture in a flask. As the mixture boils, the vapor rises, is cooled in a condenser, and then collected as a liquid distillate. This method is effective when separating components with significantly different boiling points (ideally a difference of at least 70-80°C).
Limitations of Simple Distillation:
-
Inefficient separation of close-boiling liquids: The primary drawback of simple distillation is its inability to effectively separate liquids with boiling points that are close. The vapor produced will contain a mixture of both components, leading to a distillate that is not pure. The separation achieved is limited and often results in a distillate that still contains a considerable amount of the lower-boiling component.
-
Loss of volatile components: Some components might evaporate before reaching the boiling point, leading to losses during the process. This is particularly problematic when dealing with highly volatile substances.
-
Longer distillation times: Reaching high purity levels often requires multiple distillation cycles, extending the overall process time and decreasing efficiency.
Suitable Applications of Simple Distillation:
Despite its limitations, simple distillation finds its niche in specific applications:
-
Separating liquids with vastly different boiling points: When the boiling points of the components differ substantially, simple distillation can provide a reasonably pure distillate with minimal effort. A classic example is separating water (boiling point 100°C) from salt (non-volatile).
-
Small-scale preparations: For small-scale laboratory experiments and preparations, the simplicity and low cost of simple distillation make it a convenient method.
Fractional Distillation: The Superior Choice for Complex Separations
Fractional distillation, in contrast to simple distillation, utilizes a fractionating column to significantly enhance the separation efficiency. The column provides a large surface area for repeated vaporization and condensation cycles, leading to a more gradual separation of components.
The Fractionating Column: The Key to Enhanced Separation
The fractionating column is filled with packing materials (like glass beads, metal helices, or specialized packing) which increase the surface area available for vapor-liquid equilibrium. As the vapor rises through the column, it repeatedly condenses and re-vaporizes. Each vaporization-condensation cycle leads to a gradual enrichment of the higher-boiling component in the liquid phase and the lower-boiling component in the vapor phase. This stepwise process results in a much purer distillate compared to simple distillation.
Advantages of Fractional Distillation over Simple Distillation:
-
Efficient separation of close-boiling liquids: This is the primary advantage. The repeated vaporization-condensation cycles in the fractionating column effectively separate liquids with boiling points that are close together, achieving a much higher level of purity than simple distillation.
-
Higher purity of distillate: Fractional distillation produces a distillate with significantly higher purity, often exceeding 99% for many mixtures.
-
Reduced loss of volatile components: The controlled vaporization and condensation within the column minimize the loss of volatile compounds.
-
Shorter distillation times (for the same purity level): Although the initial setup might take slightly longer, the overall distillation time can be shorter compared to performing multiple simple distillations to reach the same level of purity.
-
Improved resolution: Fractional distillation provides better resolution between components with similar boiling points, leading to sharper separation.
Detailed Mechanism of Fractional Distillation:
The process starts with heating the mixture. The vapor rises through the fractionating column. Initially, the vapor is richer in the more volatile component. As the vapor rises and cools, some of it condenses on the packing material. This liquid is then reheated by the rising hot vapor, causing it to re-vaporize. This repeated cycle enriches the vapor in the more volatile component and the liquid in the less volatile component. This process continues, with the vapor becoming increasingly enriched in the more volatile component as it travels upwards, leading to a sharp separation at the top of the column. The distillate collected at the top is enriched in the most volatile component, while the residue in the flask is enriched in the least volatile component.
Practical Applications of Fractional Distillation:
Fractional distillation is a workhorse technique in numerous industries:
-
Petroleum refining: Crude oil is a complex mixture of hydrocarbons. Fractional distillation is the primary method used to separate crude oil into its various fractions, such as gasoline, kerosene, diesel fuel, and lubricating oil. The different fractions have different boiling point ranges, which are effectively separated through fractional distillation.
-
Chemical industry: Fractional distillation is used to purify and separate various chemical compounds, especially those with relatively close boiling points. Examples include separating isomers, alcohols, and organic solvents.
-
Liquor production: The production of distilled spirits like whiskey, vodka, and gin uses fractional distillation to separate ethanol from other components in fermented mixtures. The quality and flavor of the final product are significantly influenced by the fractional distillation process.
-
Air separation: Fractional distillation is used to separate the components of air, such as nitrogen, oxygen, and argon. These gases have different boiling points and are separated in large-scale industrial plants using cryogenic fractional distillation.
Comparing Simple and Fractional Distillation: A Summary Table
| Feature | Simple Distillation | Fractional Distillation |
|---|---|---|
| Efficiency | Low, particularly for close-boiling liquids | High, even for close-boiling liquids |
| Purity | Low | High |
| Boiling Point Difference | Large (ideally >70-80°C) | Can handle smaller differences |
| Apparatus | Simple, less expensive | More complex, more expensive |
| Time | Can be longer for high purity | Generally shorter for high purity (compared to multiple simple distillations) |
| Applications | Liquids with vastly different boiling points | Close-boiling liquids, complex mixtures |
Conclusion: The Irreplaceable Role of Fractional Distillation
In conclusion, while simple distillation serves a purpose in specific applications where components have widely differing boiling points, fractional distillation reigns supreme when it comes to separating mixtures with similar boiling points. Its ability to achieve high purity, even with closely related components, makes it an indispensable technique in various industries, ensuring the production of high-quality chemicals, fuels, and gases. The added complexity of the apparatus is far outweighed by the enhanced efficiency and purity of the separation achieved through fractional distillation. Understanding the distinctions between these two techniques is crucial for choosing the appropriate method for a given separation challenge, leading to efficient and successful outcomes.
Latest Posts
Latest Posts
-
Type 1 And 2 Errors Examples
Mar 23, 2025
-
What Are The Principles Of Science
Mar 23, 2025
-
Does Electronegativity Decrease Down A Group
Mar 23, 2025
-
Derive Stefans Law From Plancks Radiation Law
Mar 23, 2025
-
A Buffer Is A Substance That
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Why Is Fractional Distillation Better Than Simple . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
