How Many Valence Electrons Are In Neon
Muz Play
Mar 22, 2025 · 6 min read
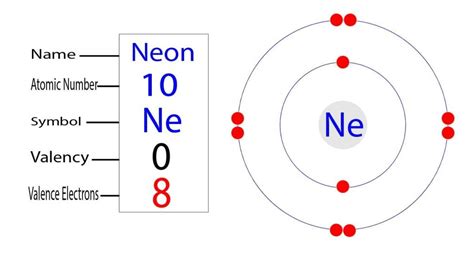
Table of Contents
How Many Valence Electrons Are in Neon? A Deep Dive into Atomic Structure
Neon, the vibrant gas that illuminates our signs and plays a crucial role in various technologies, holds a fascinating place in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is key to grasping its chemical behavior and its unique properties. This article delves deep into the world of neon, explaining not just the answer to the question "How many valence electrons are in neon?", but also the broader concepts of atomic structure, electron shells, and the significance of valence electrons in determining chemical reactivity.
Understanding Atomic Structure: The Foundation of Neon's Behavior
Before we pinpoint the number of valence electrons in neon, let's establish a foundational understanding of atomic structure. An atom, the basic building block of matter, consists of a central nucleus containing positively charged protons and neutral neutrons. Surrounding the nucleus is a cloud of negatively charged electrons, arranged in specific energy levels or shells.
These electron shells are not randomly populated. Electrons occupy shells according to a set of rules dictated by quantum mechanics. The first shell, closest to the nucleus, can hold a maximum of two electrons. The second shell can hold up to eight electrons, and subsequent shells can accommodate even more. The arrangement of electrons in these shells determines an atom's chemical properties and reactivity.
Electron Shells and Subshells: A Closer Look
Within each electron shell, there are subshells, designated by the letters s, p, d, and f. Each subshell can hold a specific number of electrons:
- s subshell: Holds a maximum of 2 electrons.
- p subshell: Holds a maximum of 6 electrons.
- d subshell: Holds a maximum of 10 electrons.
- f subshell: Holds a maximum of 14 electrons.
The filling of these subshells follows the Aufbau principle, which dictates that electrons fill the lowest energy levels first. This principle, along with Hund's rule and the Pauli exclusion principle, governs the electronic configuration of atoms.
Neon's Electronic Configuration: Unveiling the Valence Electrons
Neon (Ne), with an atomic number of 10, possesses 10 protons in its nucleus and, in its neutral state, 10 electrons orbiting the nucleus. To determine the number of valence electrons, we need to understand its electronic configuration.
The electronic configuration of neon is 1s²2s²2p⁶. Let's break this down:
- 1s²: Two electrons occupy the first shell's s subshell.
- 2s²: Two electrons occupy the second shell's s subshell.
- 2p⁶: Six electrons occupy the second shell's p subshell.
The outermost shell of an atom is known as the valence shell, and the electrons in this shell are called valence electrons. In neon's case, the second shell is the outermost shell, containing a total of eight electrons (2 from the 2s subshell and 6 from the 2p subshell).
Therefore, the answer to the question "How many valence electrons are in neon?" is definitively eight.
The Significance of Eight Valence Electrons: The Octet Rule
Neon's eight valence electrons are crucial in understanding its chemical behavior. The octet rule, a fundamental principle in chemistry, states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight electrons in their valence shell. This stable configuration resembles that of the noble gases, which are exceptionally unreactive.
Neon, already possessing a full octet of valence electrons, is exceptionally stable and unreactive. This is why neon is classified as a noble gas and exhibits minimal tendency to form chemical bonds with other elements. It is this characteristic that makes neon so valuable in applications where inertness is crucial.
Comparing Neon's Valence Electrons to Other Elements
Comparing neon's valence electron count to other elements helps illustrate the importance of this number in determining chemical reactivity. Consider the following examples:
- Sodium (Na): Sodium has one valence electron. It readily loses this electron to achieve a stable octet, forming a positive ion (Na⁺).
- Chlorine (Cl): Chlorine has seven valence electrons. It readily gains one electron to achieve a stable octet, forming a negative ion (Cl⁻).
- Oxygen (O): Oxygen has six valence electrons. It commonly shares two electrons to achieve a stable octet, forming covalent bonds.
Neon, unlike these elements, doesn't need to gain, lose, or share electrons to achieve stability. Its full valence shell makes it chemically inert.
Applications of Neon's Inertness: From Lighting to Lasers
Neon's exceptional inertness makes it invaluable in various applications, leveraging its resistance to chemical reactions:
-
Neon lighting: The characteristic orange-red glow of neon signs is produced by passing an electric current through neon gas. The excited neon atoms emit light as they return to their ground state. This inertness prevents unwanted chemical reactions that could compromise the lamp's longevity.
-
Helium-neon lasers: The combination of neon and helium gases is used in helium-neon lasers, producing a red laser beam. The inertness of neon ensures the stability and reliability of the laser.
-
Cryogenics: Neon's low boiling point allows its use in cryogenic applications, where extremely low temperatures are required. Its inert nature is essential to prevent unwanted reactions with other substances.
-
Diving mixtures: In specialized deep-sea diving, neon is sometimes used as a component of breathing mixtures. Its low density and inertness help reduce the risk of decompression sickness.
These diverse applications highlight the importance of understanding neon's atomic structure and the implications of its eight valence electrons.
Beyond the Octet Rule: Advanced Concepts
While the octet rule provides a useful framework for understanding chemical bonding, it's important to acknowledge its limitations. Elements beyond the second row of the periodic table can accommodate more than eight valence electrons, exhibiting expanded octets. However, neon, being in the second row, firmly adheres to the octet rule.
The precise arrangement of electrons in neon's valence shell contributes to its stable, spherical electron cloud, further enhancing its inertness and contributing to its unique properties. The intricacies of quantum mechanics and electron-electron interactions are crucial for a complete understanding of this stability.
Conclusion: The Significance of Neon's Eight Valence Electrons
This in-depth exploration has answered the question "How many valence electrons are in neon?" definitively: eight. This number is not just a numerical value; it is the cornerstone of neon's unique chemical behavior and its wide-ranging applications. The full valence shell, conforming to the octet rule, explains neon's exceptional stability and inertness, making it a valuable element in various technologies and scientific endeavors. Understanding the atomic structure and electron configuration of elements like neon is essential to appreciate the fundamental principles of chemistry and the remarkable diversity of the periodic table. From illuminating our cities to powering lasers, neon's eight valence electrons have a significant impact on our world.
Latest Posts
Latest Posts
-
What Is The Difference Between Actual Yield And Theoretical Yield
Mar 22, 2025
-
Compared With Solid Ionic Compounds Solid Molecular Compounds Generally
Mar 22, 2025
-
Water Is Pure Substance Or Mixture
Mar 22, 2025
-
What Is A Complex Fraction In Math
Mar 22, 2025
-
Ba Oh 2 Strong Or Weak
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Neon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
