What Is The Electron Configuration For Lithium
Muz Play
Mar 22, 2025 · 5 min read
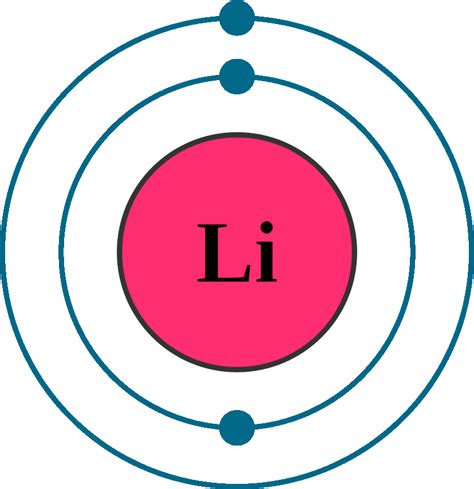
Table of Contents
What is the Electron Configuration for Lithium? A Deep Dive into Atomic Structure
Lithium, the lightest alkali metal, holds a fascinating position in the periodic table. Understanding its electron configuration is key to comprehending its unique chemical and physical properties. This article will delve deep into the electron configuration of lithium, exploring the underlying principles of atomic structure and the significance of this arrangement in determining lithium's behavior. We'll also touch upon related concepts like orbital diagrams, quantum numbers, and the exceptions to the rules.
Understanding Electron Configuration
Before we dive into the specifics of lithium, let's establish a foundational understanding of electron configuration. Simply put, an electron configuration describes the arrangement of electrons in the different energy levels (shells) and sublevels (subshells) within an atom. This arrangement is governed by the principles of quantum mechanics and dictates how an atom will interact with other atoms, forming molecules and influencing its chemical properties.
Electrons occupy orbitals within these sublevels. An orbital is a region of space where there's a high probability of finding an electron. Each orbital can hold a maximum of two electrons, according to the Pauli Exclusion Principle.
The key to understanding electron configurations lies in the Aufbau principle, which states that electrons fill the lowest energy levels first. This filling order is often depicted using the Aufbau diagram or a periodic table, which helps visualize the sequence of filling orbitals.
The Electron Configuration of Lithium (Li)
Lithium, with an atomic number of 3, possesses three electrons. To determine its electron configuration, we follow the Aufbau principle and the rules for filling orbitals:
- First shell (n=1): This shell contains only the s subshell, which can hold a maximum of two electrons. Therefore, the first two electrons of lithium fill this 1s orbital.
- Second shell (n=2): This shell contains both s and p subshells. The 2s subshell, which can hold up to two electrons, receives the third electron of lithium.
Therefore, the complete electron configuration of lithium is 1s²2s¹.
This concise notation tells us that:
- Two electrons occupy the 1s orbital.
- One electron occupies the 2s orbital.
Visualizing Electron Configuration: Orbital Diagrams
While the electron configuration provides a concise summary, orbital diagrams offer a more visual representation. These diagrams use boxes to represent orbitals and arrows to represent electrons. Each box can hold a maximum of two arrows, with arrows pointing in opposite directions to represent electrons with opposite spins (Pauli Exclusion Principle).
The orbital diagram for lithium is:
1s: ↑↓ 2s: ↑
This visually confirms that the 1s orbital is filled with two electrons, while the 2s orbital contains only one.
Quantum Numbers and Their Role
The electron configuration is intrinsically linked to the four quantum numbers that describe the state of an electron within an atom:
-
Principal Quantum Number (n): This number defines the energy level or shell (n = 1, 2, 3...). For lithium, the electrons are found in n=1 and n=2 shells.
-
Azimuthal Quantum Number (l): This number defines the subshell (l = 0 for s, l = 1 for p, l = 2 for d, etc.). Lithium's electrons are in the s subshell (l=0).
-
Magnetic Quantum Number (ml): This number specifies the orbital within a subshell (-l ≤ ml ≤ +l). The s subshell has only one orbital (ml = 0).
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron (+1/2 or -1/2, often represented by ↑ and ↓). Lithium's electrons have both spin up and spin down.
Understanding these quantum numbers is crucial for a complete grasp of the atomic structure and electron configuration of lithium and other elements.
Significance of Lithium's Electron Configuration
Lithium's electron configuration, 1s²2s¹, is pivotal in understanding its chemical reactivity and properties:
-
One Valence Electron: The outermost shell (valence shell) contains only one electron in the 2s orbital. This single valence electron is easily lost, making lithium highly reactive and prone to forming a +1 cation (Li⁺). This single valence electron is the key factor behind lithium's properties.
-
Alkali Metal Behavior: This readily available valence electron makes lithium a highly reactive alkali metal. It readily loses this electron to achieve a stable noble gas configuration (like Helium, 1s²), which is a driving force in chemical bonding.
-
Ionic Bonding: Due to its tendency to lose an electron, lithium primarily forms ionic bonds with nonmetals. It readily transfers its valence electron to electronegative elements like chlorine, oxygen, and fluorine to form ionic compounds like lithium chloride (LiCl), lithium oxide (Li₂O), and lithium fluoride (LiF).
-
Metallic Bonding: Lithium also exhibits metallic bonding, where valence electrons are delocalized throughout the metal lattice, contributing to its characteristic properties like conductivity and malleability.
-
Applications: The unique properties stemming from its electron configuration make lithium essential in various applications, including batteries (lithium-ion batteries), ceramics, and lubricants.
Exceptions to the Aufbau Principle
While the Aufbau principle provides a useful framework for predicting electron configurations, there are exceptions. These exceptions typically arise in transition metals and some other elements due to subtle energy differences between orbitals. However, lithium's electron configuration follows the Aufbau principle neatly; there are no exceptions in this case.
Conclusion
The electron configuration of lithium, 1s²2s¹, is not simply a list of numbers; it's a fundamental description of its atomic structure and the key to understanding its chemical behavior. This seemingly simple configuration dictates its reactivity, bonding characteristics, and ultimately, its numerous applications in various fields. Understanding lithium's electron configuration provides a solid foundation for grasping the principles of atomic structure and the periodic trends observed across the periodic table. By grasping this core concept, we can begin to appreciate the intricate relationship between an element's electronic structure and its macroscopic properties. Further exploration into quantum mechanics and atomic physics will provide an even deeper understanding of the fascinating world of atomic structure and the behaviors of individual elements. The seemingly simple configuration of lithium opens a door to a wealth of scientific knowledge and technological applications.
Latest Posts
Latest Posts
-
What Is A Positively Charged Ion Called
Mar 22, 2025
-
Systems Of Linear Equations In Three Variables
Mar 22, 2025
-
What Is Involved In Making A Smear
Mar 22, 2025
-
Proving The Fundamental Theorem Of Calculus
Mar 22, 2025
-
Muscle Cells Use Lactic Acid Fermentation To
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Lithium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
