What Is A Positively Charged Ion Called
Muz Play
Mar 22, 2025 · 7 min read
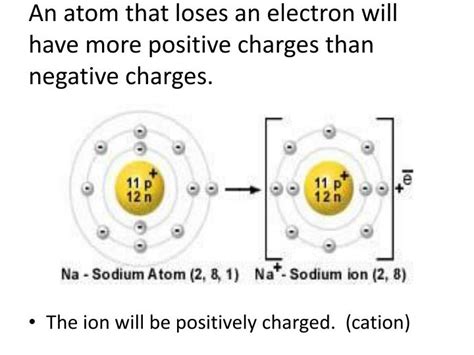
Table of Contents
What is a Positively Charged Ion Called? A Deep Dive into Cations
Have you ever wondered about the tiny particles that make up everything around us? At the heart of matter lies the atom, and within the atom, we find charged particles called ions. This article delves into the fascinating world of ions, specifically focusing on positively charged ions, also known as cations. We'll explore their formation, properties, roles in various processes, and their significance in different fields of science.
Understanding Ions: The Foundation of Charge
Before we dive into the specifics of positively charged ions, let's establish a fundamental understanding of ions themselves. An ion is an atom or molecule that carries an electric charge. This charge arises from an imbalance in the number of protons (positively charged particles) and electrons (negatively charged particles) within the atom or molecule.
Atoms, in their neutral state, possess an equal number of protons and electrons, resulting in a net charge of zero. However, under certain conditions, atoms can gain or lose electrons, disrupting this balance and creating an ion.
-
Cations: When an atom loses one or more electrons, it becomes positively charged because the number of protons now exceeds the number of electrons. This positively charged ion is called a cation. The word "cation" is derived from the Greek word "kata," meaning "down," referring to the movement of cations towards the cathode (the negatively charged electrode) in an electric field.
-
Anions: Conversely, when an atom gains one or more electrons, it becomes negatively charged because the number of electrons now exceeds the number of protons. This negatively charged ion is called an anion. The term "anion" is derived from the Greek word "ana," meaning "up," indicating the movement of anions towards the anode (the positively charged electrode) in an electric field.
The Formation of Cations: The Process of Ionization
The process of forming cations involves the loss of electrons from an atom. This can occur through several mechanisms:
1. Electrostatic Interaction:
Atoms with low ionization energies readily lose electrons when interacting with highly electronegative atoms or molecules. This often happens in chemical reactions where one atom is more likely to give up electrons to achieve a more stable electron configuration (usually a full outer electron shell). A classic example is the formation of sodium ions (Na⁺) from sodium atoms (Na). Sodium, with one electron in its outermost shell, readily loses this electron to achieve a stable octet configuration, becoming a positively charged cation.
2. Thermal Ionization:
High temperatures can provide sufficient energy to overcome the attractive force between the nucleus and electrons, causing the atom to lose one or more electrons and become a cation. This process is prevalent in stars, plasma, and high-temperature environments. The high kinetic energy of the atoms at high temperatures facilitates electron ejection.
3. Photoionization:
When an atom absorbs a photon (a quantum of light) with energy greater than its ionization energy, it can eject an electron, resulting in the formation of a cation. The energy of the photon must be sufficient to overcome the binding energy of the electron to the atom. This is a crucial process in various astronomical phenomena and is used in techniques like photoelectron spectroscopy.
4. Chemical Reactions:
Many chemical reactions involve the transfer of electrons between atoms, resulting in the formation of cations and anions. Oxidation-reduction (redox) reactions are prime examples, where one atom undergoes oxidation (loss of electrons) while another undergoes reduction (gain of electrons). The atom that loses electrons forms a cation, becoming oxidized.
Properties of Cations: Size, Charge, and Reactivity
The properties of cations are significantly different from their parent atoms. Several key properties are:
-
Smaller Size: Cations are generally smaller than their parent atoms because they have lost one or more electrons, reducing the electron cloud's size. The reduced electron-electron repulsion leads to a tighter pull by the nucleus.
-
Positive Charge: The defining characteristic of a cation is its positive charge, which is determined by the number of electrons lost. For instance, a sodium ion (Na⁺) has a +1 charge, while a magnesium ion (Mg²⁺) has a +2 charge.
-
Reactivity: The reactivity of a cation depends on its charge and size. Highly charged and small cations tend to be more reactive due to their higher charge density. They have a stronger ability to attract electrons from other atoms or molecules.
-
Electrostatic Interactions: The positive charge of cations allows them to participate in electrostatic interactions with anions and other charged species. These interactions are fundamental in the formation of ionic compounds and play a vital role in many chemical and biological processes.
The Significance of Cations in Various Fields
Cations play crucial roles in various fields, including:
1. Chemistry:
-
Ionic Compounds: Cations form the basis of numerous ionic compounds. These compounds are formed through the electrostatic attraction between cations and anions. Examples include sodium chloride (NaCl), calcium carbonate (CaCO₃), and potassium iodide (KI).
-
Redox Reactions: Cations are central to oxidation-reduction reactions, acting as electron acceptors (oxidizing agents) or electron donors (reducing agents) depending on the specific reaction.
-
Catalysis: Certain cations act as catalysts, accelerating chemical reactions without being consumed in the process.
2. Biology:
-
Biological Processes: Many biological processes rely on the presence of specific cations. For example, calcium ions (Ca²⁺) are essential for muscle contraction, nerve impulse transmission, and blood clotting. Sodium ions (Na⁺) and potassium ions (K⁺) are crucial for maintaining cell membrane potential and nerve impulse transmission. Magnesium ions (Mg²⁺) are vital cofactors in many enzyme reactions.
-
Electrolyte Balance: The balance of cations (and anions) in bodily fluids is crucial for maintaining proper physiological function. Electrolyte imbalances can lead to various health problems.
-
Mineral Nutrition: Plants require various cations, such as potassium (K⁺), calcium (Ca²⁺), and magnesium (Mg²⁺), for healthy growth and development.
3. Materials Science:
-
Materials Properties: The properties of many materials are significantly influenced by the presence of cations. For instance, the strength and conductivity of ceramics and metals can be altered by changing the cation composition.
-
Crystal Structure: Cations play a critical role in determining the crystal structure of many materials. The arrangement of cations and anions within the crystal lattice dictates the material's overall properties.
4. Environmental Science:
-
Water Quality: The concentration of various cations in water is a key indicator of water quality. High concentrations of certain cations can make water unsuitable for drinking or other purposes.
-
Soil Chemistry: Cations in the soil affect soil fertility and plant growth. The availability of essential cations is crucial for plant nutrition.
5. Medicine:
-
Electrolyte Therapy: In medicine, electrolyte imbalances are often treated by administering solutions containing specific cations.
-
Diagnostic Tools: The levels of various cations in blood and other bodily fluids can be measured to diagnose various medical conditions.
Examples of Common Cations
Let's look at some examples of commonly encountered cations:
-
Alkali Metal Cations: These include lithium (Li⁺), sodium (Na⁺), potassium (K⁺), rubidium (Rb⁺), and cesium (Cs⁺). They typically have a +1 charge.
-
Alkaline Earth Metal Cations: These include beryllium (Be²⁺), magnesium (Mg²⁺), calcium (Ca²⁺), strontium (Sr²⁺), and barium (Ba²⁺). They typically have a +2 charge.
-
Transition Metal Cations: Transition metals can form cations with various charges, such as iron (Fe²⁺, Fe³⁺), copper (Cu⁺, Cu²⁺), and zinc (Zn²⁺).
-
Other Cations: Many other elements can form cations, including ammonium (NH₄⁺), hydronium (H₃O⁺), and many more complex organic cations.
Conclusion: The Ubiquitous Role of Cations
Positively charged ions, or cations, are fundamental building blocks of matter and play essential roles in countless natural and technological processes. From the formation of ionic compounds to their crucial involvement in biological systems and the properties of materials, cations are ubiquitous in the world around us. Understanding their properties and behavior is crucial in various scientific disciplines, contributing to advancements in chemistry, biology, medicine, materials science, and environmental science. The study of cations continues to be an active and important area of research, uncovering new insights into the fundamental workings of the universe and offering exciting possibilities for technological innovation.
Latest Posts
Latest Posts
-
Why Is Fractional Distillation Better Than Simple
Mar 22, 2025
-
Standard Gibbs Free Energy Of Formation Table
Mar 22, 2025
-
Density Of Water At Temperature Chart
Mar 22, 2025
-
How To Succeed In Nursing School Pdf
Mar 22, 2025
-
What Is The Properties Of Gases
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Is A Positively Charged Ion Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
