What Is The Difference Between Lactic Acid And Alcoholic Fermentation
Muz Play
Apr 01, 2025 · 6 min read
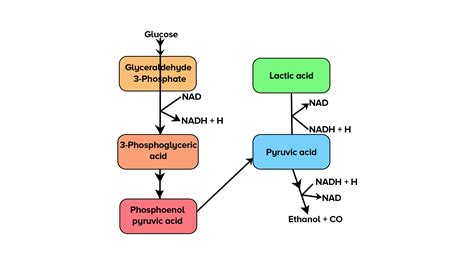
Table of Contents
Lactic Acid vs. Alcoholic Fermentation: A Deep Dive into Anaerobic Respiration
Cellular respiration, the process by which cells break down glucose to release energy, typically occurs in the presence of oxygen (aerobic respiration). However, when oxygen is scarce, organisms resort to anaerobic respiration, which yields less energy but allows survival in oxygen-deprived environments. Two prominent examples of anaerobic respiration are lactic acid fermentation and alcoholic fermentation. While both processes share the common goal of generating ATP (adenosine triphosphate) without oxygen, they differ significantly in their end products, metabolic pathways, and the organisms that utilize them. This article delves into the specifics of each process, highlighting their key distinctions and exploring their significance in various biological contexts.
Understanding Anaerobic Respiration: The Oxygen-Free Energy Source
Before diving into the specifics of lactic acid and alcoholic fermentation, it's crucial to understand the broader context of anaerobic respiration. This metabolic pathway is essential for organisms living in environments lacking sufficient oxygen. Instead of utilizing the electron transport chain, the primary energy-generating system in aerobic respiration, anaerobic respiration relies on alternative electron acceptors to produce ATP. This results in a significantly lower ATP yield compared to aerobic respiration, but it allows for the continued generation of energy, even in the absence of oxygen.
The lack of oxygen significantly impacts the fate of pyruvate, the key intermediate molecule produced during glycolysis (the initial stage of glucose breakdown). In aerobic respiration, pyruvate enters the mitochondria and is further oxidized through the Krebs cycle and the electron transport chain. In contrast, in anaerobic respiration, pyruvate undergoes different metabolic fates depending on the specific type of fermentation. This leads us to the core differences between lactic acid and alcoholic fermentation.
Lactic Acid Fermentation: The Muscle's Energy Secret
Lactic acid fermentation is a relatively simple anaerobic process that converts glucose into lactic acid. This process is primarily employed by certain bacteria (like Lactobacillus) and animals, particularly in muscle cells during periods of intense activity. When oxygen supply is insufficient to meet the energy demands, muscles switch to lactic acid fermentation to generate ATP quickly.
The Lactic Acid Fermentation Pathway: A Step-by-Step Look
-
Glycolysis: Glucose is broken down into two molecules of pyruvate. This step produces a net gain of 2 ATP molecules and 2 NADH molecules. NADH is a crucial electron carrier.
-
Pyruvate Reduction: Crucially, the pyruvate molecules are then reduced by NADH. NADH donates its electrons to pyruvate, converting it into lactic acid. This step is catalyzed by the enzyme lactate dehydrogenase. The regeneration of NAD+ (the oxidized form of NADH) is vital; it allows glycolysis to continue. Without this regeneration, glycolysis would halt due to the lack of available NAD+.
Organisms Utilizing Lactic Acid Fermentation: A Diverse Group
The versatility of lactic acid fermentation is evident in its widespread use across various organisms.
-
Animals: During strenuous exercise, when oxygen demand exceeds supply, muscle cells switch to lactic acid fermentation to maintain ATP production. The accumulation of lactic acid contributes to muscle fatigue and soreness.
-
Bacteria: Lactic acid bacteria are used extensively in food production. They are responsible for the souring of milk (yogurt, cheese production), the fermentation of sauerkraut, and the pickling of vegetables. These bacteria thrive in anaerobic conditions and produce lactic acid as a byproduct, imparting a characteristic sour taste and preserving the food.
Advantages and Disadvantages of Lactic Acid Fermentation
Advantages:
- Rapid ATP production: Provides a quick source of energy when oxygen is limited.
- Simple pathway: Requires fewer enzymes and metabolic steps compared to aerobic respiration.
- Preservative effect: In food production, lactic acid acts as a natural preservative, inhibiting the growth of spoilage organisms.
Disadvantages:
- Low ATP yield: Produces only 2 ATP molecules per glucose molecule, far less than aerobic respiration.
- Acid buildup: Accumulation of lactic acid can lead to muscle fatigue, soreness, and potentially more serious conditions in some cases.
- Limited applicability: Not suitable for long-term energy production due to the limited ATP yield.
Alcoholic Fermentation: The Yeast's Contribution to Beverage Production
Alcoholic fermentation, in contrast to lactic acid fermentation, produces ethanol (alcohol) and carbon dioxide as byproducts. This process is primarily carried out by yeast, single-celled fungi, and some bacteria. It plays a significant role in various industries, particularly in the production of alcoholic beverages and bread making.
The Alcoholic Fermentation Pathway: A Detailed Overview
-
Glycolysis: Similar to lactic acid fermentation, alcoholic fermentation begins with glycolysis, breaking down glucose into two pyruvate molecules, producing 2 ATP and 2 NADH.
-
Pyruvate Decarboxylation: Pyruvate is then decarboxylated (a carbon dioxide molecule is removed), converting it into acetaldehyde. This step is catalyzed by pyruvate decarboxylase.
-
Acetaldehyde Reduction: Acetaldehyde is reduced by NADH, converting it into ethanol. This reaction regenerates NAD+, ensuring the continued operation of glycolysis. Alcohol dehydrogenase catalyzes this crucial step.
Organisms Utilizing Alcoholic Fermentation: A Specialized Niche
The primary organisms responsible for alcoholic fermentation are yeasts, particularly species of Saccharomyces. These fungi thrive in anaerobic environments and have evolved specialized enzymes to efficiently carry out this process. Other microorganisms, including some bacteria, can also perform alcoholic fermentation, but yeasts are most commonly associated with it.
Applications of Alcoholic Fermentation: Beyond Beverages
Alcoholic fermentation has far-reaching applications, impacting several industries:
-
Beverage production: The fermentation of grapes (wine), barley (beer), and other grains (distilled spirits) relies on alcoholic fermentation to produce the characteristic alcohol content. The specific type of yeast, fermentation conditions, and other factors influence the final flavor profile of the beverage.
-
Bread making: Yeast is used in bread making to produce carbon dioxide. This gas causes the dough to rise, creating a lighter and more palatable product. The ethanol produced evaporates during baking.
-
Biofuel production: Alcoholic fermentation is being explored as a means of producing bioethanol, a renewable fuel source. This involves fermenting plant biomass (e.g., corn, sugarcane) to generate ethanol, which can be used as a fuel additive or as a standalone fuel.
Advantages and Disadvantages of Alcoholic Fermentation
Advantages:
- Ethanol production: Generates ethanol, a valuable compound with applications in beverages, biofuels, and other industries.
- Carbon dioxide production: The release of carbon dioxide is utilized in bread making to achieve dough rising.
- Relatively simple pathway: Similar to lactic acid fermentation, it involves relatively simple metabolic steps.
Disadvantages:
- Low ATP yield: Similar to lactic acid fermentation, only 2 ATP molecules are produced per glucose molecule.
- Ethanol toxicity: High concentrations of ethanol can be toxic to cells and organisms.
- Limited applicability: Not suitable for all organisms due to the potential toxicity of ethanol.
Key Differences: A Comparative Table
| Feature | Lactic Acid Fermentation | Alcoholic Fermentation |
|---|---|---|
| End Product | Lactic acid | Ethanol and carbon dioxide |
| Organisms | Animals (muscle cells), bacteria | Yeast, some bacteria |
| Enzyme(s) | Lactate dehydrogenase | Pyruvate decarboxylase, alcohol dehydrogenase |
| Intermediate | Pyruvate | Acetaldehyde |
| ATP Yield | 2 ATP per glucose molecule | 2 ATP per glucose molecule |
| Industrial Use | Food preservation, yogurt, cheese making | Alcoholic beverages, bread making, biofuels |
Conclusion: Two Sides of the Same Coin
Lactic acid and alcoholic fermentation, while both categorized under anaerobic respiration, represent distinct metabolic pathways with unique characteristics, end products, and applications. Understanding these differences is vital in appreciating the diverse strategies organisms employ to generate energy in oxygen-limited environments. From the preservation of food to the production of alcoholic beverages and biofuels, these processes play crucial roles in various aspects of human life and the broader ecosystem. Their significance extends beyond simple energy production, encompassing various biological and industrial applications, making them fascinating topics for continued research and exploration.
Latest Posts
Latest Posts
-
Molecules Consisting Only Of Carbon And Hydrogen Are Called
Apr 02, 2025
-
How To Read A Solubility Curve
Apr 02, 2025
-
Difference Between Fractional And Simple Distillation
Apr 02, 2025
-
What Are The Components Of A Language
Apr 02, 2025
-
How To Orthogonally Diagonalize A Matrix
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Lactic Acid And Alcoholic Fermentation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
