What Is The Difference Between Stereoisomers And Constitutional Isomers
Muz Play
Mar 15, 2025 · 6 min read
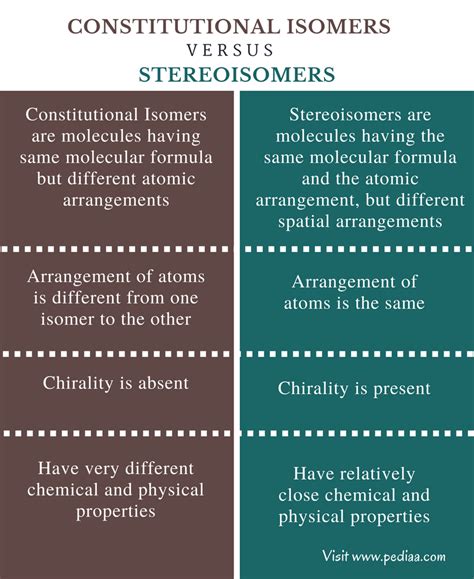
Table of Contents
Delving into the Differences: Stereoisomers vs. Constitutional Isomers
Understanding the nuances of isomerism is crucial for anyone studying organic chemistry. Isomers are molecules that share the same molecular formula but differ in their structural arrangement. This seemingly simple definition, however, masks a rich and complex world of isomeric variations. Two major categories dominate this landscape: constitutional isomers and stereoisomers. While both share the same molecular formula, their differences in connectivity and spatial arrangement lead to vastly different chemical and physical properties. This article will delve deep into the distinction between these two types of isomers, providing clear examples and elucidating the key features that separate them.
Constitutional Isomers: A Difference in Connectivity
Constitutional isomers, also known as structural isomers, represent the most fundamental form of isomerism. The defining characteristic of constitutional isomers is a difference in the connectivity of their atoms. This means that the atoms are bonded together in a different order, leading to distinct molecules with different chemical and physical properties. Think of it like using the same set of LEGO bricks to build two entirely different structures – the bricks (atoms) are the same, but the way they're connected results in unique creations.
Types of Constitutional Isomers:
Several subtypes fall under the umbrella of constitutional isomerism:
-
Chain Isomers: These isomers differ in the arrangement of their carbon chain. A straight chain can be rearranged into a branched chain, resulting in different isomers. For example, butane (C₄H₁₀) exists as both a straight-chain n-butane and a branched-chain isobutane. This difference in carbon chain structure directly influences their boiling points and reactivity.
-
Positional Isomers: These isomers have the same carbon skeleton but differ in the position of a substituent group (an atom or group of atoms attached to the main chain). For instance, 1-chloropropane and 2-chloropropane are positional isomers. The chlorine atom is attached to different carbon atoms, leading to variations in their chemical behavior.
-
Functional Group Isomers: These isomers possess the same molecular formula but contain different functional groups. Functional groups are specific groups of atoms within a molecule that determine its chemical properties. For example, ethanol (CH₃CH₂OH) and dimethyl ether (CH₃OCH₃) are functional group isomers; both have the formula C₂H₆O, but ethanol has an alcohol functional group (-OH), while dimethyl ether has an ether functional group (-O-). This difference leads to significant variations in their reactivity and properties.
Examples of Constitutional Isomers:
Let's explore some concrete examples to solidify our understanding:
-
Pentane (C₅H₁₂): Pentane exhibits three constitutional isomers: n-pentane (straight chain), isopentane (methylbutane), and neopentane (dimethylpropane). Each has a different boiling point and reactivity.
-
C₄H₈O: This molecular formula allows for several constitutional isomers, including butanal (an aldehyde), butanone (a ketone), and 2-methylpropen-1-ol (an unsaturated alcohol). These isomers have distinctly different functional groups, leading to diverse chemical properties.
-
C₅H₁₀: This formula can represent various constitutional isomers, including pentenes (alkenes with a double bond in different positions) and cyclopentane (a cyclic alkane). This highlights the impact of connectivity on molecular structure and characteristics.
The key takeaway for constitutional isomers is the variation in atom connectivity. This leads to significant differences in their physical and chemical properties, including boiling points, melting points, reactivity, and spectroscopic characteristics.
Stereoisomers: A Difference in Spatial Arrangement
Stereoisomers share the same molecular formula and the same connectivity of atoms, but they differ in the spatial arrangement of their atoms. They are essentially different three-dimensional arrangements of the same molecule. This subtle but crucial difference can have a profound impact on the molecule's properties, including its reactivity, biological activity, and even its smell or taste.
Types of Stereoisomers:
Two main types of stereoisomers exist:
-
Enantiomers: These are non-superimposable mirror images of each other. They are like left and right hands – they are similar but not identical and cannot be overlaid perfectly. Enantiomers possess a chiral center (usually a carbon atom bonded to four different groups) and exhibit optical activity, meaning they rotate plane-polarized light in opposite directions.
-
Diastereomers: These are stereoisomers that are not mirror images of each other. They possess multiple chiral centers, and the different spatial arrangements lead to various diastereomers. Diastereomers can have different physical and chemical properties. A special type of diastereomer is a geometric isomer, which arises due to restricted rotation around a double bond or in cyclic compounds. Geometric isomers are often referred to as cis-trans isomers (or E-Z isomers using the Cahn-Ingold-Prelog priority rules).
Examples of Stereoisomers:
-
2-Butanol (C₄H₁₀O): This molecule has a chiral center, resulting in two enantiomers, (R)-2-butanol and (S)-2-butanol. These enantiomers have identical physical properties (except for the rotation of polarized light), but they can interact differently with other chiral molecules, such as enzymes in the body.
-
1,2-Dichlorocyclopropane (C₃H₄Cl₂): This molecule displays cis-trans isomerism due to the ring structure. The cis isomer has both chlorine atoms on the same side of the ring, while the trans isomer has them on opposite sides. This difference in spatial arrangement affects their dipole moments and reactivity.
-
2,3-Dibromobutane (C₄H₈Br₂): This molecule possesses two chiral centers and therefore has four stereoisomers: two pairs of enantiomers that are also diastereomers to each other. These isomers have varying physical and chemical properties, impacting their reactivity and behavior in different chemical environments.
Distinguishing Between Enantiomers and Diastereomers:
The distinction between enantiomers and diastereomers is crucial. Enantiomers are mirror images, while diastereomers are not. This fundamental difference impacts their properties. Enantiomers typically have identical physical properties (except for optical rotation), whereas diastereomers usually exhibit different physical and chemical properties like melting points, boiling points, and reactivity.
Summary Table: Key Differences Between Constitutional and Stereoisomers
| Feature | Constitutional Isomers | Stereoisomers |
|---|---|---|
| Molecular Formula | Same | Same |
| Connectivity | Different | Same |
| Spatial Arrangement | May or may not be different | Different |
| Types | Chain, positional, functional group | Enantiomers, diastereomers (geometric isomers included) |
| Properties | Different physical and chemical properties | Enantiomers: similar physical properties, different optical activity; Diastereomers: different physical and chemical properties |
Conclusion: The Importance of Isomerism in Chemistry
The distinction between constitutional and stereoisomers is fundamental to understanding the vast diversity of molecules in organic chemistry and beyond. The subtle differences in connectivity and spatial arrangement have profound impacts on the properties and behaviors of molecules, impacting everything from their reactivity to their biological activity and applications in various fields, including medicine, materials science, and pharmaceuticals. A thorough grasp of isomerism is crucial for anyone seeking a deep understanding of molecular structure and its relationship to function. Understanding these concepts provides a robust foundation for more advanced studies in organic chemistry and related disciplines.
Latest Posts
Latest Posts
-
Fourier Transform Of A Differential Equation
Mar 15, 2025
-
Which One Neutral Charge Proton Or Neutron
Mar 15, 2025
-
A Solids Volume And Shape Is Defintie
Mar 15, 2025
-
How Many Protons Does Iodine Have
Mar 15, 2025
-
If The Finches On The Galapagos Islands
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Stereoisomers And Constitutional Isomers . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
