What Is The Electron Configuration For Ne
Muz Play
Apr 01, 2025 · 7 min read
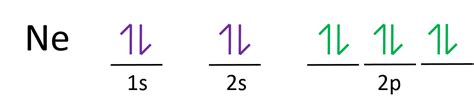
Table of Contents
What is the Electron Configuration for Neon? Understanding Atomic Structure and Stability
Neon, a noble gas with the symbol Ne and atomic number 10, is renowned for its exceptional stability and inertness. This inherent stability stems directly from its electron configuration, a fundamental concept in chemistry that dictates an atom's chemical properties and reactivity. This article delves deep into neon's electron configuration, explaining its significance, how it's determined, and its implications for neon's unique characteristics. We will also explore related concepts like orbital filling rules, quantum numbers, and the periodic table trends that help us understand neon's position within the broader context of chemical elements.
Understanding Electron Configuration
The electron configuration of an atom describes the arrangement of electrons within its electron shells and subshells. It essentially tells us which energy levels and orbitals the electrons occupy. This arrangement is crucial because it dictates how an atom will interact with other atoms, forming chemical bonds and determining its overall reactivity. Electrons are arranged according to specific rules, primarily governed by the principles of quantum mechanics.
Quantum Numbers and Orbitals
Before diving into neon's specific configuration, let's briefly review the key quantum numbers that define an electron's state within an atom:
-
Principal Quantum Number (n): This number designates the electron shell or energy level. It's a positive integer (n = 1, 2, 3, etc.), with higher values indicating greater energy and distance from the nucleus.
-
Azimuthal Quantum Number (l): This number specifies the subshell or orbital type within a shell. It ranges from 0 to n-1. Each value of l corresponds to a specific orbital shape:
- l = 0: s orbital (spherical)
- l = 1: p orbital (dumbbell-shaped)
- l = 2: d orbital (more complex shapes)
- l = 3: f orbital (even more complex shapes)
-
Magnetic Quantum Number (ml): This number describes the orientation of the orbital in space. It ranges from -l to +l, including 0. For example, a p subshell (l=1) has three orbitals (ml = -1, 0, +1).
-
Spin Quantum Number (ms): This number indicates the intrinsic angular momentum of the electron, often represented as +1/2 (spin up) or -1/2 (spin down). The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. This means each orbital can hold a maximum of two electrons with opposite spins.
Determining Neon's Electron Configuration
Neon (Ne) has an atomic number of 10, meaning it has 10 protons and 10 electrons in a neutral atom. To determine its electron configuration, we follow the Aufbau principle, which states that electrons fill the lowest energy levels first. We also consider Hund's rule, which dictates that electrons will individually occupy each orbital within a subshell before pairing up.
The order of filling orbitals is typically represented by the following sequence:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p…
Following this order, we can distribute neon's 10 electrons:
- 1s²: The first shell (n=1) contains only an s subshell, which can hold up to two electrons.
- 2s²: The second shell (n=2) also has an s subshell, holding another two electrons.
- 2p⁶: The second shell also contains a p subshell, which can hold up to six electrons (three orbitals, each with two electrons).
Therefore, the complete electron configuration for neon is 1s²2s²2p⁶. This configuration represents a filled shell and subshell structure.
Significance of Neon's Electron Configuration
Neon's completely filled electron shells are the key to understanding its chemical properties. Atoms strive for stability, often achieved by having a full valence shell (the outermost electron shell). Neon's filled 2s and 2p subshells create a very stable electron configuration, making it exceptionally unreactive. It rarely forms chemical bonds with other elements, hence its classification as a noble gas.
Noble Gases and Chemical Inertness
The noble gases (Helium, Neon, Argon, Krypton, Xenon, and Radon) all share the characteristic of having completely filled valence electron shells. This full valence shell provides exceptional stability, resulting in their extremely low reactivity. They are often referred to as inert gases because they rarely participate in chemical reactions under normal conditions.
Implications for Neon's Properties
Neon's stable electron configuration contributes to several of its observable properties:
- Inertness: As mentioned, neon's low reactivity is a direct consequence of its stable electron configuration. It doesn't readily form compounds with other elements.
- Gaseous State: The weak interatomic forces between neon atoms, due to their stable electron configurations, result in neon existing as a gas at room temperature.
- Low Boiling Point: The weak interatomic forces also lead to a very low boiling point for neon, requiring minimal energy to overcome these forces and transition from liquid to gas.
- Electrical Conductivity: While neon itself is a poor conductor of electricity under normal conditions, it can become conductive when ionized (losing electrons), creating a plasma that emits light. This property is used in neon lights, where an electric current passes through neon gas, exciting its electrons and causing them to emit characteristic red-orange light.
Neon's Position in the Periodic Table
Neon's electron configuration perfectly aligns with its position in the periodic table. It is located in Group 18 (also known as Group VIIIA or the noble gases) and Period 2. Its position reflects its filled valence shell and the resulting chemical inertness shared by other elements in this group. The periodic table organizes elements based on their electron configurations and recurring chemical properties, offering a powerful tool for predicting the behavior of elements based on their positions.
Beyond the Basic Electron Configuration: Excited States
While the ground state electron configuration (1s²2s²2p⁶) represents neon's most stable state, it can be excited by supplying energy, such as by absorbing photons. This excitation promotes an electron to a higher energy level, resulting in an excited state electron configuration. These excited states are typically short-lived, and the electron will quickly return to the ground state, emitting energy in the form of photons (light). This process is responsible for the characteristic emission spectrum of neon, often seen in neon lights.
For example, one possible excited state for neon might involve promoting an electron from the 2p orbital to a higher energy 3s orbital. This would result in an electron configuration like 1s²2s²2p⁵3s¹. However, this excited state is unstable and will quickly transition back to the ground state, releasing energy as light. The specific wavelengths of light emitted during these transitions are characteristic of neon and form its unique spectral fingerprint.
Applications of Neon's Properties
Neon's unique properties, largely a result of its stable electron configuration, are harnessed in various applications:
-
Neon Lighting: The characteristic red-orange glow of neon light is widely used for signage and decorative lighting. Different colors can be achieved by using other gases or coating the tube with different phosphors.
-
Cryogenics: Neon's low boiling point allows for its use as a cryogenic refrigerant, especially in applications requiring extremely low temperatures.
-
Lasers: Neon is used in some types of gas lasers, where the excited state of neon atoms contributes to light amplification.
Conclusion
Neon's electron configuration, 1s²2s²2p⁶, is the fundamental factor that determines its chemical inertness and unique properties. This stable configuration, with completely filled electron shells, makes neon exceptionally unreactive and contributes to its use in various applications. Understanding neon's electron configuration not only illuminates its behavior but also provides insight into the broader principles of atomic structure and the periodic trends that govern chemical properties. The principles discussed here – the Aufbau principle, Hund's rule, and quantum numbers – are crucial for comprehending the electron configurations of all elements, demonstrating the underlying order and predictability within the seemingly complex world of atomic structure.
Latest Posts
Latest Posts
-
Rate Of Change Positive Or Negative
Apr 02, 2025
-
Activation Energy For The Forward Reaction
Apr 02, 2025
-
Base Excision Repair Vs Mismatch Repair
Apr 02, 2025
-
Is Argon Metal Nonmetal Or Metalloid
Apr 02, 2025
-
What Is A Limiting Amino Acid In A Protein
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Ne . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
