What Is The Electron Configuration Of B
Muz Play
Mar 27, 2025 · 6 min read
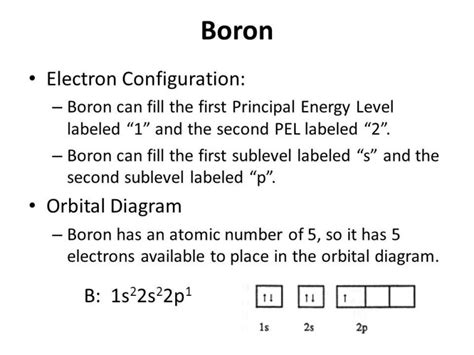
Table of Contents
What is the Electron Configuration of Boron (B)? A Deep Dive into Atomic Structure
Boron, a metalloid element with the symbol B and atomic number 5, holds a significant place in chemistry and material science. Understanding its electron configuration is key to grasping its chemical behavior and properties. This article will delve deep into the electron configuration of boron, exploring its implications for bonding, reactivity, and overall chemical characteristics. We'll also touch upon the principles governing electron configuration and how they apply to other elements within the periodic table.
Understanding Electron Configuration
Before we delve into boron's specific electron configuration, let's establish a foundational understanding of the concept. Electron configuration describes the arrangement of electrons in the various energy levels (shells) and sublevels (subshells) within an atom. This arrangement dictates how an atom will interact with other atoms, forming chemical bonds and influencing its properties.
Electrons occupy orbitals, which are regions of space around the nucleus where there's a high probability of finding an electron. Each orbital can hold a maximum of two electrons, following the Pauli Exclusion Principle. These orbitals are grouped into subshells, denoted by the letters s, p, d, and f. The subshells are further organized into shells, represented by the principal quantum number (n), which indicates the energy level.
The Aufbau principle dictates the filling order of electrons into these orbitals and subshells. Electrons first fill the lowest energy levels before moving to higher energy levels. The Hund's rule states that electrons will individually occupy each orbital within a subshell before pairing up. Finally, the Pauli exclusion principle ensures that no two electrons within an atom can have the same set of four quantum numbers (n, l, ml, and ms).
Determining the Electron Configuration of Boron (B)
Boron, with an atomic number of 5, possesses five protons in its nucleus and, in its neutral state, five electrons orbiting the nucleus. Using the Aufbau principle, we can determine the electron configuration as follows:
- 1s² 2s² 2p¹
Let's break down this configuration:
- 1s²: The first shell (n=1) contains only one subshell, the 's' subshell. This 's' subshell can hold up to two electrons. Boron's two lowest energy electrons fill this subshell completely.
- 2s²: The second shell (n=2) also contains an 's' subshell, which again can hold up to two electrons. These two electrons fill this subshell completely.
- 2p¹: The second shell also contains three 'p' orbitals, each capable of holding up to two electrons, for a total of six electrons. However, in Boron, only one electron occupies one of the 2p orbitals. This leaves two 2p orbitals empty.
Therefore, the complete electron configuration of boron is 1s² 2s² 2p¹. This configuration highlights that boron has three valence electrons – the electrons in the outermost shell (n=2). These valence electrons play a crucial role in boron's chemical bonding and reactivity.
Implications of Boron's Electron Configuration
Boron's electron configuration significantly influences its chemical behavior and properties. The presence of three valence electrons means boron tends to form covalent bonds, sharing electrons with other atoms to achieve a more stable electron configuration. This is in contrast to elements with completely filled outer shells, which are generally less reactive.
Bonding and Reactivity
Boron readily forms covalent bonds, most notably with elements such as hydrogen, oxygen, and halogens. For example, boron forms compounds like boric acid (H₃BO₃) and boron trifluoride (BF₃). The tendency to form three covalent bonds arises from its three valence electrons. It strives to complete its outermost shell, resembling the stable configuration of a noble gas.
While it doesn't readily lose its three electrons to form a +3 ion (like aluminum in group 13), it can participate in ionic bonding under specific circumstances, though this is less common compared to covalent bonding.
Chemical Properties Stemming from Electron Configuration
The unique electron configuration of boron results in several key chemical properties:
- Amphoteric Nature: Boron exhibits amphoteric behavior, meaning it can act as both an acid and a base. This is influenced by the presence of partially filled p-orbitals in its valence shell.
- High Melting and Boiling Points: Although a metalloid, boron possesses relatively high melting and boiling points compared to other metalloids. This stems from the strong covalent bonding in its various forms.
- Formation of Borides: Boron forms stable compounds called borides with many transition metals. This reflects the ability of boron to engage in strong covalent bonding with metals.
- Occurrence in various forms: Boron's electron configuration allows it to exist in various allotropic forms, including amorphous boron and crystalline boron.
Comparing Boron's Electron Configuration to Other Elements
Let's compare boron's electron configuration to other elements within its group (Group 13 or IIIA) and neighboring elements:
-
Aluminum (Al): Aluminum (atomic number 13) has an electron configuration of 1s² 2s² 2p⁶ 3s² 3p¹. While both boron and aluminum have three valence electrons, aluminum's larger size and lower electronegativity lead to differences in their chemical reactivity. Aluminum is significantly more reactive than boron.
-
Carbon (C): Carbon (atomic number 6) has an electron configuration of 1s² 2s² 2p². Carbon is a non-metal, and despite having one fewer valence electron than boron, it exhibits drastically different chemical behavior. The differences arise from its tendency to form multiple bonds and complex structures.
-
Beryllium (Be): Beryllium (atomic number 4), located in Group 2, has an electron configuration of 1s² 2s². With only two valence electrons, it differs sharply from boron in its chemical properties, typically forming 2+ ions.
These comparisons highlight how subtle changes in electron configuration significantly impact an element's chemical properties and reactivity.
Advanced Concepts and Further Exploration
The simple electron configuration provides a fundamental understanding of boron's behavior. However, more advanced concepts further refine our understanding:
-
Orbital Hybridization: Boron's bonding often involves orbital hybridization, where atomic orbitals combine to form new hybrid orbitals with different shapes and energies, optimizing bonding interactions. For instance, in BF₃, boron utilizes sp² hybridization.
-
Molecular Orbital Theory: A more sophisticated model, molecular orbital theory, describes the behavior of electrons in molecules, providing a deeper understanding of bonding and molecular properties than simple electron configuration.
-
Spectroscopy: Various spectroscopic techniques (such as UV-Vis, NMR, etc.) provide experimental evidence supporting the theoretical understanding of electron configuration and energy levels within boron-containing compounds.
Conclusion: The Significance of Electron Configuration in Understanding Boron
The electron configuration of boron, 1s² 2s² 2p¹, provides a foundational understanding of its chemical behavior. Its three valence electrons dictate its tendency to form covalent bonds, its amphoteric nature, and its role in various compounds and materials. By understanding its electron configuration, we gain valuable insights into its unique properties and applications in diverse fields ranging from materials science to medicine. The principles governing electron configuration extend beyond boron, providing a framework for understanding the periodic trends and chemical behavior of all elements in the periodic table. This deeper understanding allows us to predict and manipulate chemical interactions for various applications. Further exploration of advanced concepts like orbital hybridization and molecular orbital theory offers even more nuanced insight into the fascinating world of boron chemistry.
Latest Posts
Latest Posts
-
How Does Fermentation Allow Glycolysis To Continue
Mar 30, 2025
-
Which Subatomic Particle Carries A Positive Charge
Mar 30, 2025
-
Signs A Chemical Reaction Has Occurred
Mar 30, 2025
-
Duties Of An Agent In Law
Mar 30, 2025
-
What Is The Symbol For Population Variance
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of B . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
