What Is The Electron Configureation For Lithium
Muz Play
Mar 17, 2025 · 5 min read
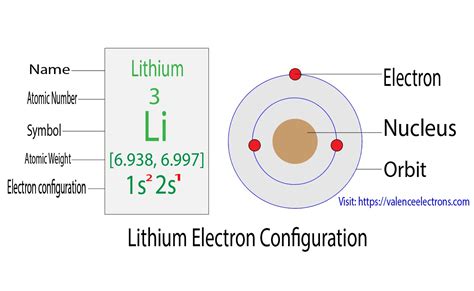
Table of Contents
What is the Electron Configuration for Lithium? A Deep Dive into Atomic Structure
Lithium, the lightest of the alkali metals, holds a significant place in chemistry and beyond. Understanding its electron configuration is key to grasping its unique properties and reactivity. This article will delve deep into the electron configuration of lithium, exploring its derivation, implications, and broader context within atomic structure and periodic trends.
Understanding Electron Configuration
Before we dive into the specifics of lithium, let's establish a foundational understanding of electron configuration. Electron configuration describes the arrangement of electrons in the various energy levels (shells) and sublevels (subshells) within an atom. This arrangement dictates an atom's chemical behavior, its reactivity, and its place within the periodic table.
Electrons occupy orbitals, which are regions of space around the nucleus where there's a high probability of finding an electron. Each orbital can hold a maximum of two electrons, according to the Pauli Exclusion Principle. Orbitals are grouped into subshells (s, p, d, f), each with a specific number of orbitals and, consequently, a maximum number of electrons.
- The s subshell: Holds a maximum of 2 electrons in one orbital.
- The p subshell: Holds a maximum of 6 electrons in three orbitals.
- The d subshell: Holds a maximum of 10 electrons in five orbitals.
- The f subshell: Holds a maximum of 14 electrons in seven orbitals.
These subshells are arranged in energy levels or shells, designated by principal quantum numbers (n = 1, 2, 3, etc.). Lower energy levels are filled first, following the Aufbau principle (building-up principle). The order of filling is not always strictly sequential due to the relative energies of subshells. Hund's rule dictates that electrons will individually occupy orbitals within a subshell before pairing up.
Deriving Lithium's Electron Configuration
Lithium (Li) has an atomic number of 3, meaning it possesses 3 protons and, in its neutral state, 3 electrons. To determine its electron configuration, we follow the Aufbau principle and Hund's rule.
-
First shell (n=1): The lowest energy level, this shell contains only the 1s subshell, which can hold a maximum of two electrons. Therefore, both of lithium's first two electrons fill this subshell.
-
Second shell (n=2): The next energy level contains the 2s and 2p subshells. After filling the 1s subshell, the remaining electron goes into the 2s subshell. The 2p subshell remains unoccupied.
Therefore, the complete electron configuration for lithium is 1s²2s¹.
This configuration succinctly describes the arrangement of lithium's three electrons: two in the 1s orbital and one in the 2s orbital. The superscript numbers indicate the number of electrons in each subshell.
Visualizing Lithium's Electron Configuration
We can visualize lithium's electron configuration using orbital diagrams. An orbital diagram uses boxes to represent orbitals and arrows to represent electrons. For lithium:
- 1s subshell: Two boxes representing the 1s orbital, each filled with an upward and downward arrow representing the two electrons.
1s: ↑↓ - 2s subshell: One box representing the 2s orbital, with a single upward arrow representing the remaining electron.
2s: ↑
Implications of Lithium's Electron Configuration
Lithium's electron configuration (1s²2s¹) directly influences its chemical and physical properties:
-
Reactivity: The single electron in the 2s orbital is relatively loosely held. This makes lithium highly reactive, readily losing this electron to form a +1 cation (Li⁺). This explains its characteristic reactivity with water and other substances.
-
Ionic Bonding: The tendency to lose an electron and form a stable cation with a full outermost shell (octet) leads to lithium's participation in ionic bonding, commonly found in its compounds like lithium chloride (LiCl) and lithium oxide (Li₂O).
-
Metallic Character: Lithium's electron configuration contributes to its metallic character. The ease with which it loses an electron facilitates the formation of a "sea" of delocalized electrons, characteristic of metals and responsible for properties like electrical conductivity.
-
First Ionization Energy: The energy required to remove the outermost electron (2s¹) is relatively low, consistent with its high reactivity. This low first ionization energy is a direct consequence of its electron configuration and the shielding effect of the inner electrons.
Lithium's Position in the Periodic Table and Trends
Lithium's electron configuration perfectly aligns with its position in the periodic table. As an alkali metal, it belongs to Group 1 (IA), characterized by having one valence electron – the electron in the outermost shell. This single valence electron explains the consistent +1 oxidation state of lithium and its characteristic reactivity.
Comparing Lithium to other Alkali Metals
The other alkali metals (sodium, potassium, rubidium, cesium, and francium) all share a similar electron configuration pattern, with a single electron in their outermost s subshell. However, the number of inner shells increases down the group, leading to subtle but significant differences in their properties. For example, while all alkali metals are reactive, the reactivity generally increases as we go down the group due to the increasing distance of the valence electron from the nucleus.
Lithium's Applications and Significance
Lithium's unique properties, stemming directly from its electron configuration, have led to a wide range of applications:
-
Batteries: Lithium-ion batteries, leveraging lithium's ability to readily lose and gain electrons, are ubiquitous in portable electronics, electric vehicles, and grid-scale energy storage.
-
Ceramics and Glass: Lithium compounds are used in the production of various ceramics and glasses, contributing to improved properties such as strength and durability.
-
Lubricants: Lithium-based greases are employed as high-performance lubricants due to their excellent thermal stability and resistance to oxidation.
-
Medicine: Lithium salts have found use in the treatment of certain mental health conditions, although their mechanism of action is complex and not fully understood.
Conclusion: A Fundamental Understanding
The seemingly simple electron configuration of lithium (1s²2s¹) holds the key to understanding its diverse properties and its significant role in various technologies and applications. Its reactive nature, arising from the readily available valence electron, makes it crucial in battery technology and other fields. By understanding the principles of electron configuration, atomic structure, and periodic trends, we gain a deeper appreciation for the fundamental behavior of elements and their profound impact on our world. Further exploration of quantum mechanics and atomic physics reveals even greater detail concerning electron behavior and provides a more complete picture of the complexities hidden within this simple yet crucial atomic arrangement.
Latest Posts
Latest Posts
-
Fallacies Divide Into Roughly Two Kinds
Mar 17, 2025
-
An Organism That Cannot Grow Without Oxygen Is A An
Mar 17, 2025
-
Difference Between Chemical Reaction And Nuclear Reaction
Mar 17, 2025
-
Which Graph Shows Line Symmetry About The Y Axis
Mar 17, 2025
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configureation For Lithium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
