What Is The Final Electron Acceptor In Fermentation
Muz Play
Mar 25, 2025 · 6 min read
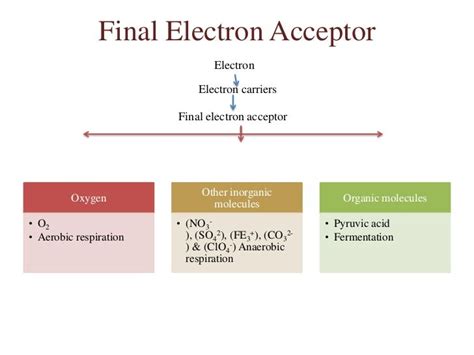
Table of Contents
What is the Final Electron Acceptor in Fermentation? Understanding Anaerobic Respiration
Fermentation, a cornerstone of anaerobic metabolism, often leaves students and researchers alike with a lingering question: what is the final electron acceptor? The answer, while seemingly simple, requires a nuanced understanding of the process itself. Unlike cellular respiration, which utilizes oxygen as the terminal electron acceptor in the electron transport chain, fermentation employs a different strategy entirely. The crucial point to grasp is that fermentation does not have a final electron acceptor in the same way as aerobic respiration. Instead, it focuses on regenerating NAD+ to sustain glycolysis, the initial step in glucose breakdown.
The Role of NAD+ and NADH in Fermentation
To understand why there's no "final electron acceptor" in the traditional sense, we need to delve into the role of nicotinamide adenine dinucleotide (NAD+). NAD+ is a crucial coenzyme in glycolysis. During glycolysis, glucose is oxidized, and electrons are transferred to NAD+, reducing it to NADH. This NADH carries high-energy electrons.
In aerobic respiration, these high-energy electrons are passed down the electron transport chain, ultimately reducing oxygen to water. This process generates a significant amount of ATP, the cell's energy currency.
However, fermentation occurs in the absence of oxygen (anaerobic conditions). Without oxygen to accept electrons at the end of the electron transport chain, the process would grind to a halt. The NADH build-up would inhibit glycolysis, halting ATP production. Fermentation's primary purpose, therefore, is to regenerate NAD+ from NADH, enabling glycolysis to continue producing a small amount of ATP.
Understanding the Different Types of Fermentation
Various types of fermentation exist, each employing a different organic molecule as an electron sink, not a final electron acceptor in the same manner as oxidative phosphorylation. These processes essentially use organic molecules to re-oxidize NADH back to NAD+. Let's explore some prominent examples:
1. Lactic Acid Fermentation: A Common Pathway
Lactic acid fermentation is perhaps the most well-known type. It occurs in muscle cells during strenuous exercise when oxygen supply is limited and in some microorganisms like Lactobacillus and Streptococcus.
In lactic acid fermentation, pyruvate, the end-product of glycolysis, acts as the electron sink. NADH donates its electrons to pyruvate, reducing it to lactate. This regenerates NAD+, allowing glycolysis to continue. The lactate itself is a byproduct and often accumulates, contributing to muscle fatigue or giving yogurt its characteristic tartness. Note: Pyruvate isn't a final electron acceptor in the way oxygen is; it’s merely a temporary electron recipient, crucial for NAD+ regeneration.
2. Alcoholic Fermentation: The Basis of Brewing and Baking
Alcoholic fermentation is a crucial process in brewing and baking. Yeasts, such as Saccharomyces cerevisiae, employ this pathway. Here, pyruvate undergoes a two-step conversion.
First, pyruvate is decarboxylated, releasing carbon dioxide (CO2), forming acetaldehyde. Then, NADH reduces acetaldehyde to ethanol, regenerating NAD+. Again, acetaldehyde, not a terminal electron acceptor, accepts electrons from NADH. The end products, ethanol and CO2, are responsible for the alcoholic beverages and leavened bread, respectively. This process highlights again that regeneration of NAD+ is the core goal, not a reduction of a terminal electron acceptor with the production of a proton gradient.
3. Propionic Acid Fermentation: A Unique Pathway
Propionic acid fermentation is another anaerobic pathway employed by bacteria in the genus Propionibacterium. This process is important in Swiss cheese production, contributing to its characteristic flavor and holes.
In this pathway, pyruvate is converted to propionate, acetate, and carbon dioxide. Different intermediates act as electron sinks in this complex metabolic pathway, ultimately regenerating NAD+. The diverse end products contribute to the complex flavor profiles of the cheese. Again, the critical component is the regeneration of NAD+, enabling continued glycolysis, not the final electron acceptor.
4. Butyric Acid Fermentation: The Clostridia's Role
Clostridium bacteria employ butyric acid fermentation, producing butyric acid (butanoic acid) as the primary end product. This process involves multiple steps and intermediates, with the ultimate goal, again, being the regeneration of NAD+. The butyric acid produced contributes to the characteristic odor of rancid butter and some industrial products. The lack of a traditional final electron acceptor underscores the core function of this pathway.
5. Mixed Acid Fermentation: A Complex Variety
Certain bacteria utilize mixed acid fermentation, producing a variety of acidic end products, including lactic acid, acetic acid, succinic acid, formic acid, ethanol, and carbon dioxide. This complexity reflects a range of electron sink molecules used to regenerate NAD+. The mixture of acids contributes to the unique properties of fermented products.
Distinguishing Fermentation from Anaerobic Respiration
It's crucial to distinguish fermentation from anaerobic respiration. While both occur in the absence of oxygen, they differ significantly in their electron acceptor and energy yield.
-
Fermentation: Uses an organic molecule (like pyruvate or acetaldehyde) as an electron sink to regenerate NAD+, producing a small amount of ATP (only from glycolysis). No electron transport chain is involved.
-
Anaerobic Respiration: Uses an inorganic molecule (like sulfate, nitrate, or fumarate) as a final electron acceptor in an electron transport chain, generating a larger amount of ATP than fermentation. An electron transport chain and proton gradient are involved.
The key difference lies in the nature of the electron acceptor and the involvement of an electron transport chain. Fermentation lacks a terminal electron acceptor in the traditional sense, whereas anaerobic respiration uses an inorganic molecule to serve that role, leading to ATP production through oxidative phosphorylation.
Why the Confusion Around "Final Electron Acceptor" in Fermentation?
The confusion surrounding the "final electron acceptor" in fermentation stems from the inaccurate analogy to aerobic respiration. While aerobic respiration has a clear final electron acceptor (oxygen), fermentation doesn't function in the same manner. Its primary objective is not to reduce a final electron acceptor but to regenerate NAD+ for the continuation of glycolysis. The organic molecules involved are best described as electron sinks or reducing agents rather than final electron acceptors within the framework of the electron transport chain.
The Importance of NAD+ Regeneration in Fermentation
The central theme uniting all fermentation pathways is the crucial role of NAD+ regeneration. The production of ATP is secondary; maintaining a sufficient supply of NAD+ to fuel glycolysis is paramount for cell survival in the absence of oxygen. Without the regeneration of NAD+, glycolysis would come to a standstill, severely limiting the cell’s ability to produce energy.
Conclusion: A Functional Perspective on Fermentation
Understanding fermentation requires shifting our perspective from a quest for a "final electron acceptor" to a focus on the critical role of NAD+ regeneration. The various types of fermentation demonstrate the diverse ways organisms can achieve this vital goal, utilizing a range of organic molecules as electron sinks. This adaptation allows for the continuation of glycolysis and a small but essential energy supply in anaerobic environments. Instead of focusing on the misleading "final electron acceptor" concept, understanding the central importance of NAD+ regeneration provides a clearer and more accurate understanding of this vital metabolic process. This functional approach enhances our appreciation of the ingenuity of life's strategies for energy production in challenging conditions.
Latest Posts
Latest Posts
-
List The Three Components Of Traditional Cell Theory
Mar 25, 2025
-
What Stage Of Cellular Respiration Produces The Most Atp
Mar 25, 2025
-
Which Correctly Summarizes The Trend In Electron Affinity
Mar 25, 2025
-
Delta H Delta S Delta G Chart
Mar 25, 2025
-
A Chemical Equation Is Balanced When
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Final Electron Acceptor In Fermentation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
