What Is The Horizontal Row Of The Periodic Table Called
Muz Play
Mar 16, 2025 · 7 min read
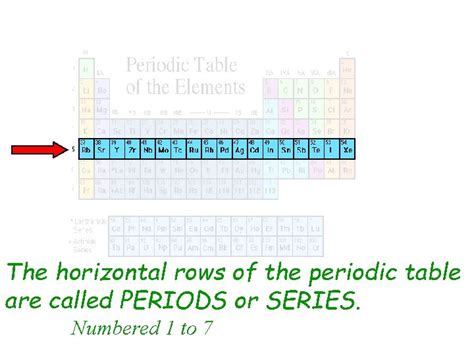
Table of Contents
What is the Horizontal Row of the Periodic Table Called? A Deep Dive into Periods and Their Significance
The periodic table, that iconic chart adorning countless science classrooms, organizes the chemical elements in a way that reveals their properties and relationships. But have you ever stopped to consider the specific names of the table's horizontal and vertical arrangements? While many are familiar with the "groups" or "families" of vertically aligned elements, the horizontal rows often get less attention. This article delves into the answer to the question: What is the horizontal row of the periodic table called? It's called a period, and understanding periods is crucial to comprehending the periodic table's structure and predictive power.
Understanding the Structure: Periods vs. Groups
The periodic table's organization is a marvel of scientific ingenuity. It's not merely a list; it's a visual representation of the periodic law, which states that the properties of elements are periodic functions of their atomic numbers. This organization manifests in two key arrangements:
-
Periods (Horizontal Rows): These rows represent elements with the same number of electron shells. As you move across a period, the atomic number increases, adding one proton and one electron. This systematic addition affects the element's electronic configuration and, consequently, its chemical and physical properties.
-
Groups or Families (Vertical Columns): These columns group elements with similar outer electron configurations, leading to similar chemical behavior. Elements within a group tend to exhibit similar reactivity and form similar types of compounds.
The interaction between periods and groups is what makes the periodic table so powerful. It allows scientists to predict the properties of an element based on its position within the table, even before the element has been fully characterized.
The Significance of Periods: A Closer Look
Each period in the periodic table corresponds to a principal energy level, or shell, in an atom's electron configuration. The first period, for instance, contains only hydrogen (H) and helium (He), both of which have electrons in the first energy level (n=1). This level can hold a maximum of two electrons.
As we move down the periodic table to subsequent periods, the number of electrons and energy levels increases. The second period (Li to Ne) contains elements with electrons filling the second energy level (n=2), which can accommodate up to eight electrons. Similarly, the third period (Na to Ar) adds electrons to the third energy level (n=3), also capable of holding eight electrons.
This pattern continues, though the maximum number of electrons in each subsequent energy level becomes increasingly complex, reflecting the intricate rules governing electron configuration in higher energy levels.
Period Length and Electron Shells
The length of each period is directly related to the number of electrons that can occupy the subshells within a given principal energy level. The first two periods are relatively short, containing only two and eight elements, respectively. This is due to the limited number of orbitals available in the s and p subshells of the first two energy levels.
Later periods become longer due to the inclusion of d and f subshells, which can accommodate additional electrons and, thus, more elements. Periods 4 and 5 have 18 elements each, encompassing the d-block elements (transition metals). Periods 6 and 7 are even longer, accommodating the f-block elements (lanthanides and actinides), which are usually displayed separately at the bottom of the periodic table for clarity.
Trends in Properties Across a Period
Moving across a period, several key properties exhibit predictable trends:
-
Atomic Radius: Generally decreases across a period. As the number of protons increases, the increased positive charge pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
-
Ionization Energy: Generally increases across a period. It becomes increasingly difficult to remove an electron from an atom as the nuclear charge increases and the electrons are held more tightly.
-
Electronegativity: Generally increases across a period. Atoms with higher electronegativity attract electrons more strongly in a chemical bond.
-
Metallic Character: Generally decreases across a period. Elements transition from metallic to non-metallic characteristics as you move from left to right.
These trends are a direct consequence of the systematic changes in electronic configuration as you proceed across a period. These predictable trends make the periodic table an invaluable tool for chemists and other scientists.
The Periods and Their Elements: A Detailed Overview
Let's examine each period in more detail, highlighting key characteristics and the elements they contain:
Period 1: Hydrogen (H) and Helium (He). These are unique elements forming the first energy level. Hydrogen is a highly reactive nonmetal, while helium is an inert noble gas.
Period 2: Lithium (Li) to Neon (Ne). This period introduces the s and p orbitals, leading to a wider range of properties. It includes alkali metals (Li, Na), alkaline earth metals (Be, Mg), and various nonmetals and a noble gas (Ne).
Period 3: Sodium (Na) to Argon (Ar). Similar to period 2, but with larger atoms and slightly different chemical properties due to the increased energy level.
Period 4: Potassium (K) to Krypton (Kr). The inclusion of the d-block (transition metals) significantly increases the period length and introduces elements with variable oxidation states and complex chemical behavior.
Period 5: Rubidium (Rb) to Xenon (Xe). Similar to period 4, showing further trends in properties.
Period 6: Cesium (Cs) to Radon (Rn). Contains the lanthanides (rare earth elements) which are placed separately below the main table due to space considerations.
Period 7: Francium (Fr) to Oganesson (Og). This period also includes the actinides, which are also placed separately. The period 7 elements are largely radioactive and synthetically produced.
Understanding the characteristics of each period and the trends in properties across each period provides a complete picture of how the periodic table arranges the elements based on their atomic structure and chemical behavior.
Beyond the Basics: Applications and Importance of Periodicity
The concept of periods and their associated properties isn't merely an academic exercise; it has profound practical applications in various fields:
-
Material Science: Understanding periodic trends allows scientists to design new materials with specific properties. For example, knowing the electronegativity of elements allows for the prediction and creation of materials with desired electrical conductivity or strength.
-
Chemistry: The periodic table is fundamental to understanding chemical reactions, bonding, and predicting the behavior of elements in different compounds.
-
Nuclear Physics: The radioactive properties of elements, particularly those in higher periods, are crucial for applications such as nuclear medicine and energy production.
-
Drug Discovery and Development: Understanding the reactivity of elements, determined by their positions in periods and groups, is critical in designing and developing new drugs.
-
Environmental Science: The periodic table aids in understanding the interactions of elements in environmental systems, pollution control, and remediation strategies.
The ability to predict the properties of an element based on its position within the periodic table – its period and group – is a testament to the power of scientific observation, organization, and the predictive capabilities of the periodic law.
Conclusion: The Enduring Importance of Periods
In conclusion, the horizontal row of the periodic table is called a period. These periods are fundamental to the organization and understanding of the periodic table, representing elements with the same number of electron shells. The predictable trends in properties across periods, alongside the similar properties of elements within groups, make the periodic table an indispensable tool for scientific investigation and technological advancement across numerous disciplines. From predicting chemical reactivity to designing new materials, the understanding of periods and their significance remains a cornerstone of modern science. Remembering that each period adds a new energy level to an atom's electron configuration provides a powerful framework for comprehending the remarkable structure and utility of the periodic table.
Latest Posts
Latest Posts
-
Cellulose Is Composed Of Monomers Of
Mar 17, 2025
-
Find The Expansion Base Of N Formula
Mar 17, 2025
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Horizontal Row Of The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
