What Is The Most Electronegative Element On The Periodic Table
Muz Play
Mar 15, 2025 · 6 min read
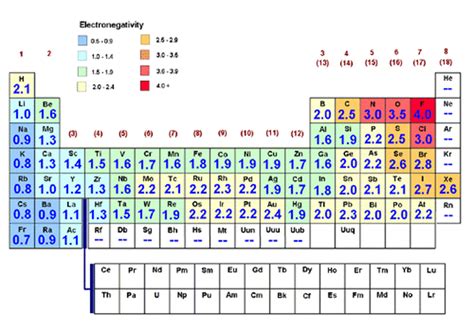
Table of Contents
What is the Most Electronegative Element on the Periodic Table?
Fluorine. The answer, simply put, is fluorine. But understanding why fluorine holds this title requires a deeper dive into the fascinating world of electronegativity and the periodic table itself. This article will explore not only the undisputed champion, fluorine, but also the underlying principles that govern electronegativity and how it influences chemical behavior.
Understanding Electronegativity: The Tug-of-War of Electrons
Electronegativity is a crucial concept in chemistry, describing an atom's ability to attract electrons within a chemical bond. Think of it as a tug-of-war: the more electronegative an atom, the stronger its pull on the shared electrons in a covalent bond. This pull isn't about outright stealing electrons (that's ionization), but about exerting a greater influence on their location. The greater the electronegativity difference between two atoms, the more polar the bond becomes, with the more electronegative atom carrying a partial negative charge (δ−) and the less electronegative atom carrying a partial positive charge (δ+).
Factors Influencing Electronegativity
Several factors contribute to an atom's electronegativity:
-
Nuclear Charge: A higher positive charge in the nucleus exerts a stronger attractive force on electrons. As you move across the periodic table (left to right), the nuclear charge increases, generally leading to higher electronegativity.
-
Atomic Radius: A smaller atomic radius means the electrons are closer to the nucleus, experiencing a stronger pull. Electronegativity generally increases as you move up a group (column) on the periodic table, as atomic radius decreases.
-
Shielding Effect: Inner electrons shield outer electrons from the full positive charge of the nucleus. The more inner electrons, the less the outer electrons "feel" the nuclear charge, reducing electronegativity.
These factors work in concert. While nuclear charge generally increases electronegativity, shielding effect can mitigate this increase. This interplay ultimately determines the electronegativity trend across the periodic table.
Fluorine: The Reigning Electronegativity Champion
Fluorine (F), located in the top right corner of the periodic table, boasts the highest electronegativity of all elements. This exceptional electronegativity stems from a powerful combination of factors:
-
High Nuclear Charge: Fluorine possesses a relatively high nuclear charge for its size, resulting in a strong pull on electrons.
-
Small Atomic Radius: Fluorine has the smallest atomic radius among all the main group elements. This proximity of the electrons to the nucleus significantly boosts its electronegativity.
-
Minimal Shielding: With only two electron shells, fluorine experiences minimal shielding effect. The outer electrons are thus exposed to a relatively high effective nuclear charge.
This potent combination of a high nuclear charge, small atomic radius, and minimal shielding makes fluorine exceptionally adept at attracting electrons in a chemical bond. Its electronegativity is so high that it forms exceptionally strong bonds with many other elements.
Comparing Fluorine to Other Highly Electronegative Elements
While fluorine reigns supreme, other elements exhibit high electronegativity. Let's compare fluorine to some of its closest competitors:
-
Oxygen (O): Oxygen is the second most electronegative element. While it has a smaller atomic radius than many elements, it also has a slightly lower nuclear charge and more shielding compared to fluorine.
-
Chlorine (Cl): Chlorine has a higher nuclear charge than oxygen but a larger atomic radius. The increased distance between the nucleus and valence electrons diminishes its electronegativity compared to fluorine.
-
Nitrogen (N): Nitrogen, while electronegative, has a lower electronegativity than oxygen due to its slightly larger atomic radius and similar shielding.
The differences in electronegativity between these elements might seem subtle, but these small variations have significant implications for the properties of the compounds they form.
The Significance of Electronegativity in Chemistry
Electronegativity is not merely an abstract concept; it plays a vital role in determining various chemical properties and behaviors:
1. Bond Polarity:
As mentioned earlier, the difference in electronegativity between two bonded atoms dictates the polarity of the bond. A large electronegativity difference leads to a polar bond, where electrons are unequally shared, resulting in partial charges on the atoms. This polarity influences the molecule's overall properties, such as its solubility, boiling point, and reactivity.
2. Bond Strength:
Greater electronegativity difference can lead to stronger bonds, particularly in ionic compounds. The stronger attraction between oppositely charged ions results in a more stable and higher melting point compound.
3. Molecular Geometry and Dipole Moment:
Polar bonds contribute to the overall dipole moment of a molecule, influencing its shape and interactions with other molecules. The symmetrical distribution of polar bonds can result in a nonpolar molecule, even if individual bonds are polar.
4. Chemical Reactivity:
Electronegativity plays a crucial role in determining the reactivity of elements and compounds. Highly electronegative elements like fluorine tend to be highly reactive because of their strong tendency to gain electrons and form stable anions.
5. Acid-Base Behavior:
Electronegativity can influence the acidic or basic character of a molecule. For example, highly electronegative atoms can draw electron density away from hydrogen atoms, making them more acidic.
Electronegativity Scales: Quantifying the Tug-of-War
Several scales have been developed to quantify electronegativity, the most widely used being the Pauling scale. This scale, proposed by Linus Pauling, assigns fluorine an electronegativity value of 4.0, setting the benchmark against which all other elements are measured. Other scales, such as the Mulliken scale and the Allred-Rochow scale, offer alternative ways to express electronegativity, but they all show the same trend: fluorine holds the top spot.
The Exceptional Reactivity of Fluorine: A Consequence of High Electronegativity
Fluorine's extremely high electronegativity directly contributes to its exceptional reactivity. It readily forms strong bonds with almost all other elements, often with highly exothermic reactions (releasing significant heat). This reactivity makes fluorine a powerful oxidizing agent, capable of oxidizing many substances that are resistant to oxidation by other elements.
Safety Considerations: Handling Fluorine
Due to its extreme reactivity, fluorine requires careful handling and special safety precautions. It readily reacts with water, many metals, and even some noble gases, making containment and manipulation challenging. Specialized equipment and training are essential when working with fluorine.
Conclusion: Fluorine's Undisputed Reign
In conclusion, fluorine's dominance in electronegativity is not just a matter of definition but a consequence of its unique atomic structure and properties. Its high nuclear charge, small atomic radius, and minimal shielding effect all combine to create an element with an unparalleled ability to attract electrons. This extreme electronegativity drives its exceptional reactivity and influences its behavior in a wide range of chemical processes, making it a pivotal element in chemistry and beyond. The understanding of electronegativity and its impact on chemical behavior is fundamental to numerous areas of chemistry, from predicting reaction pathways to designing new materials. Fluorine, the champion of electronegativity, serves as a powerful example of how subtle atomic properties can have profound effects on the macroscopic world.
Latest Posts
Latest Posts
-
Which One Neutral Charge Proton Or Neutron
Mar 15, 2025
-
A Solids Volume And Shape Is Defintie
Mar 15, 2025
-
How Many Protons Does Iodine Have
Mar 15, 2025
-
If The Finches On The Galapagos Islands
Mar 15, 2025
-
How To Find A Perpendicular Vector
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Most Electronegative Element On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
