What Is The Most Reactive Group In The Periodic Table
Muz Play
Mar 26, 2025 · 5 min read
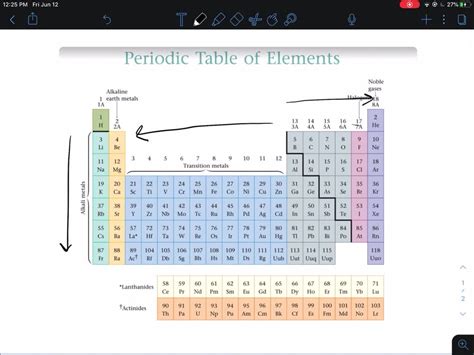
Table of Contents
What is the Most Reactive Group in the Periodic Table?
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding reactivity is crucial to predicting chemical behavior. While the question of the most reactive group is nuanced and depends on the specific context (e.g., reactivity with water, oxygen, or other elements), the alkali metals (Group 1) and halogens (Group 17) consistently vie for the title. This article will delve deep into the reactivity of these groups, exploring the underlying reasons and providing illustrative examples.
Understanding Reactivity: A Fundamental Concept
Reactivity, in simple terms, refers to an element's tendency to undergo chemical reactions. It's determined by several factors:
-
Electron Configuration: The arrangement of electrons in an atom's shells significantly impacts its reactivity. Elements strive to achieve a stable electron configuration, often resembling a noble gas (Group 18). This drive towards stability drives chemical reactions.
-
Electronegativity: This measures an atom's ability to attract electrons in a chemical bond. Highly electronegative elements readily attract electrons from less electronegative elements, forming stable compounds.
-
Ionization Energy: The energy required to remove an electron from an atom is its ionization energy. Elements with low ionization energies readily lose electrons, contributing to their reactivity.
-
Atomic Radius: The size of an atom influences its reactivity. Smaller atoms can more easily attract electrons, while larger atoms can more easily lose electrons.
The Alkali Metals (Group 1): Masters of Electron Donation
The alkali metals—lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr)—are renowned for their exceptionally high reactivity. This stems from their electron configuration: they possess a single valence electron in their outermost shell.
Why are Alkali Metals So Reactive?
-
Single Valence Electron: This single electron is relatively loosely held and easily lost. Losing this electron allows the alkali metal to achieve a stable, noble gas electron configuration.
-
Low Ionization Energy: Because the valence electron is loosely held, it requires minimal energy to remove it. This low ionization energy makes electron donation highly favorable.
-
Large Atomic Radius: As you move down Group 1, the atomic radius increases. This means the valence electron is further from the nucleus and experiences less electrostatic attraction, making it even easier to lose.
Examples of Alkali Metal Reactivity:
-
Reaction with Water: Alkali metals react vigorously with water, producing hydrogen gas and metal hydroxides. The reaction becomes increasingly violent as you move down the group. For example, lithium reacts moderately, while sodium reacts vigorously, and cesium reacts explosively. The general equation is:
2M(s) + 2H₂O(l) → 2MOH(aq) + H₂(g) -
Reaction with Halogens: Alkali metals react readily with halogens (Group 17) to form ionic compounds called alkali halides. These reactions are highly exothermic (release significant heat). For example:
2Na(s) + Cl₂(g) → 2NaCl(s)(formation of table salt) -
Reaction with Oxygen: Alkali metals react with oxygen to form oxides or peroxides. The nature of the product depends on the alkali metal and the reaction conditions.
The Halogens (Group 17): Electron Thieves
The halogens—fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At)—are another highly reactive group. Their reactivity stems from their electron configuration: they have seven valence electrons, one short of a stable noble gas configuration.
Why are Halogens So Reactive?
-
Seven Valence Electrons: Halogens strongly desire to gain one more electron to complete their octet and achieve noble gas configuration.
-
High Electronegativity: Halogens possess high electronegativity, meaning they strongly attract electrons. This allows them to readily gain electrons from other elements.
-
High Electron Affinity: Halogens have a high electron affinity, which means they release a significant amount of energy when they gain an electron. This makes electron gain energetically favorable.
Examples of Halogen Reactivity:
-
Reaction with Alkali Metals: As mentioned earlier, halogens react violently with alkali metals to form ionic compounds.
-
Reaction with Hydrogen: Halogens react with hydrogen to form hydrogen halides, which are strong acids in aqueous solution. For example:
H₂(g) + Cl₂(g) → 2HCl(g) -
Displacement Reactions: A more reactive halogen can displace a less reactive halogen from its compound. For example, chlorine can displace bromine from bromide salts:
Cl₂(g) + 2NaBr(aq) → 2NaCl(aq) + Br₂(l) -
Reactions with Other Metals: Halogens react with many other metals besides alkali metals, forming ionic halides.
Comparing Alkali Metals and Halogens: A Detailed Analysis
Both alkali metals and halogens exhibit exceptionally high reactivity, but through different mechanisms. Alkali metals readily lose electrons, while halogens readily gain electrons. The relative reactivity within each group varies, influenced by factors like atomic size and ionization/electron affinity.
Within Group 1 (Alkali Metals): Reactivity increases down the group due to the increasing atomic radius and decreasing ionization energy. Francium is the most reactive alkali metal, although its scarcity makes experimental study challenging.
Within Group 17 (Halogens): Reactivity generally decreases down the group. Fluorine, being the smallest and most electronegative, is the most reactive halogen. The larger size and decreased electronegativity of the heavier halogens make it harder for them to attract and accept an electron.
Other Reactive Groups
While alkali metals and halogens are the most commonly cited highly reactive groups, other elements also display significant reactivity. These include:
-
Alkaline Earth Metals (Group 2): These elements have two valence electrons and are less reactive than alkali metals but still readily participate in reactions.
-
Group 16 (Chalcogens): Oxygen and sulfur are highly reactive nonmetals, readily forming compounds with many other elements.
Conclusion: Context Matters
Determining the most reactive group depends heavily on the specific context. In reactions involving electron donation, alkali metals typically display higher reactivity. Conversely, in reactions involving electron gain, halogens generally exhibit greater reactivity. Both groups showcase extreme tendencies in terms of their electron configurations, making them powerful players in a vast array of chemical processes. Understanding the nuances of electron configuration, ionization energy, electronegativity, and atomic radius is key to comprehending the reactivity of elements and predicting their behavior in various chemical scenarios. The periodic table, therefore, remains an indispensable tool for exploring and understanding the intricacies of chemical reactivity.
Latest Posts
Latest Posts
-
What Are The Elements In Group 18 Called
Mar 29, 2025
-
How Do You Make A Standard Curve
Mar 29, 2025
-
Hydrogen Is A Metal Nonmetal Or Metalloid
Mar 29, 2025
-
Equation Of Tangent Line Implicit Differentiation
Mar 29, 2025
-
According To The Bronsted Lowry Definition
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Most Reactive Group In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
