What Is The Ph For A Neutral Solution
Muz Play
Mar 22, 2025 · 6 min read
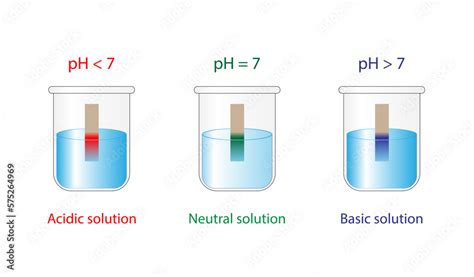
Table of Contents
What is the pH for a Neutral Solution? A Deep Dive into pH, Acidity, and Alkalinity
Understanding pH is fundamental to numerous scientific disciplines, from chemistry and biology to environmental science and medicine. This comprehensive guide delves into the intricacies of pH, focusing specifically on what constitutes a neutral solution and the implications of pH deviations. We'll explore the pH scale, the role of water in determining pH, and practical applications of pH measurement across various fields.
Understanding the pH Scale: A Measure of Acidity and Alkalinity
The pH scale is a logarithmic scale ranging from 0 to 14, used to specify the acidity or alkalinity of an aqueous solution. It's a measure of the concentration of hydrogen ions (H⁺) in a solution. The lower the pH value, the higher the concentration of H⁺ ions and the more acidic the solution. Conversely, a higher pH value indicates a lower concentration of H⁺ ions and a more alkaline (basic) solution.
Key Points of the pH Scale:
- pH 7: Neutral. This represents a perfectly balanced solution where the concentration of H⁺ ions equals the concentration of hydroxide ions (OH⁻). Pure water at 25°C (77°F) has a pH of 7.
- pH < 7: Acidic. Solutions with a pH less than 7 are considered acidic. Examples include lemon juice (pH ~2), stomach acid (pH ~1-3), and vinegar (pH ~3). The further the pH is from 7, the stronger the acid.
- pH > 7: Alkaline (Basic). Solutions with a pH greater than 7 are considered alkaline or basic. Examples include baking soda solution (pH ~8-9), household ammonia (pH ~11-12), and lye (pH ~13-14). The further the pH is from 7, the stronger the base.
It's crucial to remember that the pH scale is logarithmic. This means that a change of one pH unit represents a tenfold change in the concentration of hydrogen ions. For instance, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4, and one hundred times more acidic than a solution with a pH of 5.
The Role of Water in Determining pH: Autoionization and the Equilibrium Constant
Water itself plays a critical role in determining the pH of a solution. Water molecules undergo a process called autoionization, where a small fraction of water molecules spontaneously dissociate into hydrogen ions (H⁺) and hydroxide ions (OH⁻):
H₂O ⇌ H⁺ + OH⁻
This equilibrium is represented by the ion product constant of water (Kw):
Kw = [H⁺][OH⁻]
At 25°C, Kw has a value of approximately 1.0 × 10⁻¹⁴. In pure water, the concentration of H⁺ ions equals the concentration of OH⁻ ions, both being approximately 1.0 × 10⁻⁷ M. This leads to a pH of 7, confirming that pure water at 25°C is neutral.
However, the value of Kw, and consequently the pH of neutrality, is temperature-dependent. At higher temperatures, the autoionization of water increases, resulting in a higher Kw and a slightly lower pH of neutrality (closer to 6.14 than 7). This subtle shift is crucial in specific applications where temperature variations significantly influence the system.
Maintaining pH Neutrality: Buffers and Their Importance
Maintaining a neutral pH is often critical in biological systems and many industrial processes. Buffers are solutions that resist changes in pH when small amounts of acid or base are added. They're crucial for maintaining a stable pH environment, which is essential for various biological processes and chemical reactions.
Buffers typically consist of a weak acid and its conjugate base, or a weak base and its conjugate acid. These components work together to neutralize added H⁺ or OH⁻ ions, minimizing pH fluctuations. The effectiveness of a buffer depends on its buffer capacity and the pH range over which it operates.
Deviations from Neutrality: The Impacts of Acidity and Alkalinity
Deviations from a neutral pH, whether towards acidity or alkalinity, can have significant consequences, depending on the context.
Impacts of Acidity:
- Corrosion: Acidic solutions can corrode metals, leading to structural damage and equipment failure. This is a major concern in industrial settings and infrastructure maintenance.
- Environmental damage: Acid rain, caused by the release of sulfur dioxide and nitrogen oxides into the atmosphere, can significantly lower the pH of soil and water bodies, harming aquatic life and vegetation.
- Health effects: Exposure to highly acidic substances can cause skin burns, eye irritation, and respiratory problems. Maintaining a safe pH in industrial settings is crucial to worker safety.
Impacts of Alkalinity:
- Saponification: Strong alkaline solutions can react with fats and oils, a process known as saponification, which is used in soap making but can also be damaging to certain materials.
- Environmental damage: Highly alkaline solutions can harm aquatic organisms and alter the chemical balance of ecosystems.
- Health effects: Contact with strong alkaline solutions can cause severe skin burns and eye damage. Safe handling and disposal of alkaline substances are crucial.
Measuring pH: Techniques and Applications
Accurate pH measurement is crucial across numerous fields. Several methods exist for determining the pH of a solution:
- pH Indicators: These are substances that change color depending on the pH of the solution. Litmus paper is a common example, turning red in acidic solutions and blue in alkaline solutions. More sophisticated indicators provide a broader range of color changes, enabling more precise pH estimation.
- pH Meters: These electronic devices use a glass electrode to measure the potential difference between the solution and a reference electrode. This potential difference is directly related to the pH of the solution. pH meters are highly accurate and are widely used in laboratories and industrial settings.
- Spectrophotometry: This technique utilizes the absorption of light by specific indicators at different wavelengths to determine the pH. It's particularly useful for measuring the pH of complex solutions or those containing interfering substances.
Practical Applications of pH Measurement:
The applications of pH measurement are extensive and span many disciplines:
- Water quality monitoring: Measuring the pH of water bodies is crucial for assessing their health and suitability for drinking, irrigation, and aquatic life.
- Agriculture: Soil pH is a critical factor affecting plant growth. Farmers adjust soil pH using fertilizers and amendments to optimize crop yields.
- Food and beverage industry: pH control is crucial during food processing and preservation to ensure product quality, safety, and shelf life.
- Medicine: pH levels are closely monitored in the human body, as deviations can indicate various health conditions. Blood pH, for instance, is tightly regulated to maintain homeostasis.
- Chemical industry: pH control is essential in many chemical processes, ensuring the efficiency and safety of reactions.
Conclusion: The Significance of pH Neutrality and its Deviations
The pH of a solution, specifically whether it's neutral, acidic, or alkaline, is a fundamental property with far-reaching consequences. Understanding the pH scale, the role of water in determining pH, the importance of buffers, and the various methods for measuring pH are essential for numerous applications in science, technology, and everyday life. While a pH of 7 signifies neutrality at 25°C, the implications of deviations from this value are vast and require careful consideration in various contexts. Precise pH control and monitoring are critical for maintaining optimal conditions in diverse fields, impacting everything from environmental protection to human health.
Latest Posts
Latest Posts
-
The Crural Region Of The Body Is The
Mar 23, 2025
-
Draw The Shear And Moment Diagram
Mar 23, 2025
-
How Do You Calculate Temperature Change
Mar 23, 2025
-
What Is The Relation Between Temperature And Pressure
Mar 23, 2025
-
Find Determinant By Row Reduction To Echelon Form
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Ph For A Neutral Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
