What Is The Property Of A Base
Muz Play
Mar 21, 2025 · 6 min read
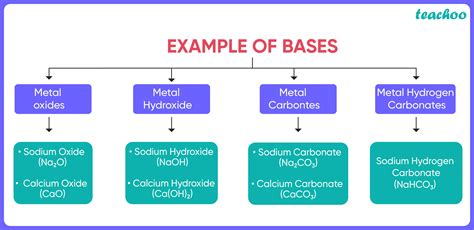
Table of Contents
What is the Property of a Base? A Comprehensive Guide
Understanding the properties of bases is fundamental to grasping many concepts in chemistry. From everyday applications like baking soda to complex industrial processes, bases play a crucial role. This comprehensive guide delves into the defining characteristics of bases, exploring their chemical properties, physical properties, and practical applications.
Defining Bases: Arrhenius, Brønsted-Lowry, and Lewis Theories
The definition of a base has evolved over time, with different theories offering nuanced perspectives.
Arrhenius Definition: The Hydroxide Ion Connection
The simplest definition comes from Svante Arrhenius. He defined a base as a substance that, when dissolved in water, increases the concentration of hydroxide ions (OH⁻). This is a straightforward definition, applicable to many common bases like sodium hydroxide (NaOH) and potassium hydroxide (KOH). These bases dissociate in water to release hydroxide ions, making the solution alkaline.
Example: NaOH(aq) → Na⁺(aq) + OH⁻(aq)
However, the Arrhenius definition is limited. It doesn't encompass all substances that exhibit basic properties, particularly those that don't contain hydroxide ions.
Brønsted-Lowry Definition: The Proton Acceptor
A more comprehensive approach is provided by the Brønsted-Lowry theory. This theory defines a base as a proton acceptor. A proton, in this context, refers to a hydrogen ion (H⁺). A Brønsted-Lowry base accepts a proton from an acid, forming a conjugate acid. This definition expands the scope of bases to include molecules and ions that don't necessarily contain hydroxide ions.
Example: Ammonia (NH₃) acts as a Brønsted-Lowry base by accepting a proton from water:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
In this reaction, NH₃ accepts a proton from H₂O, forming the ammonium ion (NH₄⁺), while water acts as an acid, donating a proton.
Lewis Definition: The Electron Pair Donor
The most general definition comes from Gilbert N. Lewis. A Lewis base is defined as an electron-pair donor. This definition encompasses a wider range of substances than the previous two, including those that don't contain hydrogen or hydroxide ions. A Lewis base donates a lone pair of electrons to a Lewis acid, which is an electron-pair acceptor.
Example: Ammonia (NH₃) acts as a Lewis base by donating its lone pair of electrons to a boron trifluoride (BF₃) molecule:
NH₃ + BF₃ → H₃N-BF₃
Here, the nitrogen atom in ammonia donates a lone pair of electrons to the boron atom in BF₃, forming a coordinate covalent bond.
Chemical Properties of Bases
Bases exhibit several key chemical properties:
1. Reaction with Acids: Neutralization
The most characteristic reaction of bases is their neutralization reaction with acids. This reaction produces water and a salt.
Example: The reaction between sodium hydroxide (a base) and hydrochloric acid (an acid):
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
This reaction is exothermic, meaning it releases heat. The heat released is often used to determine the concentration of an unknown acid or base through titration.
2. Reaction with Metals: Hydrogen Gas Evolution
Certain bases, particularly those in the form of molten hydroxides, react with certain metals, such as aluminum and zinc, to produce hydrogen gas.
Example: Reaction of sodium hydroxide with aluminum:
2Al(s) + 2NaOH(aq) + 6H₂O(l) → 2Na + 3H₂(g)
This reaction is often used to generate hydrogen gas in a laboratory setting.
3. Reaction with Metal Oxides and Non-metal Oxides: Salt Formation
Bases react with many metal oxides and non-metal oxides. With metal oxides, they form salts and water, while with non-metal oxides (acid anhydrides), they form salts.
Example:
- Reaction with a metal oxide: CaO(s) + 2HCl(aq) → CaCl₂(aq) + H₂O(l)
- Reaction with a non-metal oxide: CO₂(g) + 2NaOH(aq) → Na₂CO₃(aq) + H₂O(l)
4. Saponification: Soap Production
Bases, particularly strong bases like sodium hydroxide and potassium hydroxide, play a critical role in saponification, the process of making soap. They react with fats and oils (triglycerides) to produce soap (fatty acid salts) and glycerol.
Physical Properties of Bases
Bases also exhibit distinctive physical properties:
1. Taste: Bitter Taste
Many bases have a bitter taste. However, it's crucial to never taste chemicals to determine their properties; this is a dangerous practice.
2. Feel: Slippery or Soapy Feel
Bases often feel slippery or soapy to the touch. This is due to their reaction with the oils and proteins on the skin. Again, direct contact should be avoided to prevent skin irritation or damage.
3. pH: pH greater than 7
The most reliable way to identify a base is by measuring its pH. Bases have a pH greater than 7, with strong bases having a pH close to 14. The pH scale measures the concentration of hydrogen ions in a solution; a higher pH indicates a lower concentration of hydrogen ions and a higher concentration of hydroxide ions.
4. Electrical Conductivity: Conduct Electricity in Solution
Bases, when dissolved in water, conduct electricity. This is because they dissociate into ions, which are mobile charge carriers. The extent of conductivity depends on the strength of the base; strong bases are better conductors than weak bases.
5. Indicators: Change color of indicators
Bases can be identified using acid-base indicators, which change color depending on the pH of the solution. Common indicators include litmus paper (turns blue in the presence of a base) and phenolphthalein (turns pink in the presence of a base).
Examples of Bases
The world is full of bases, both strong and weak. Here are some common examples:
- Sodium hydroxide (NaOH): A strong base, used in many industrial processes, including soap making and drain cleaning.
- Potassium hydroxide (KOH): Another strong base, similar to sodium hydroxide in its properties and uses.
- Calcium hydroxide (Ca(OH)₂): A moderately strong base, used in the production of cement and plaster.
- Ammonia (NH₃): A weak base, used in cleaning products and as a fertilizer.
- Baking soda (Sodium bicarbonate, NaHCO₃): A weak base, commonly used in baking and as an antacid.
- Magnesium hydroxide (Mg(OH)₂): A weak base, used in antacids and laxatives.
Applications of Bases
The versatility of bases leads to a wide range of applications in various industries:
- Industrial Processes: Bases are essential in many industrial processes, including the production of soap, paper, textiles, and fertilizers.
- Food Industry: Bases are used as food additives, pH regulators, and leavening agents.
- Medicine: Bases are used in antacids to neutralize stomach acid, and as laxatives.
- Agriculture: Bases are used to adjust the pH of soil and as fertilizers.
- Environmental Applications: Bases are used in wastewater treatment and to neutralize acidic spills.
Conclusion: Understanding the Significance of Bases
Understanding the properties of bases is crucial across multiple disciplines. From their fundamental chemical behavior to their diverse applications, bases are integral to various processes in our daily lives and industrial operations. The different definitions—Arrhenius, Brønsted-Lowry, and Lewis—provide increasingly comprehensive perspectives on basicity, highlighting the diverse range of compounds exhibiting basic properties. Recognizing the physical properties and chemical reactivity of bases enables us to predict their behavior in different situations and utilize them effectively in various applications. Further exploration into the specific properties of individual bases, as well as their interactions with acids and other substances, provides a deeper understanding of chemical principles and their practical applications.
Latest Posts
Latest Posts
-
What Happens During The Reduction Stage Of The Calvin Cycle
Mar 27, 2025
-
Is Solid To Liquid Endothermic Or Exothermic
Mar 27, 2025
-
What Does A Negative Enthalpy Mean
Mar 27, 2025
-
Divides The Body Into Anterior And Posterior Portions
Mar 27, 2025
-
Claim Of Fact Value And Policy
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Is The Property Of A Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
