What Is The Relationship Between A Mole And Avogadro's Number
Muz Play
Mar 31, 2025 · 6 min read
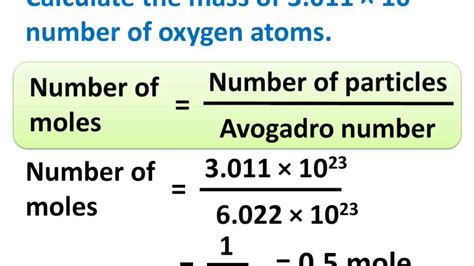
Table of Contents
What is the Relationship Between a Mole and Avogadro's Number?
Understanding the relationship between a mole and Avogadro's number is fundamental to chemistry. It's the cornerstone of stoichiometry, allowing us to bridge the gap between the macroscopic world of grams and liters and the microscopic world of atoms and molecules. This article will delve deep into this crucial concept, explaining both terms individually before exploring their interconnectedness with numerous examples and analogies.
Understanding the Mole
In everyday life, we use units like dozens (12), gross (144), or reams (500) to count items. Chemists, however, deal with incredibly large numbers of atoms and molecules. Imagine trying to count the number of atoms in a single gram of water! That's where the mole comes in.
The mole (mol) is a fundamental unit in the International System of Units (SI), representing a specific amount of substance. It's essentially a counting unit, similar to a dozen or a gross, but on a much grander scale. One mole of a substance contains Avogadro's number of constituent particles (atoms, molecules, ions, etc.).
Why Use Moles?
The mole provides a convenient way to handle the vast quantities of particles involved in chemical reactions. Instead of working with astronomically large numbers of individual atoms or molecules, we can use moles to represent these quantities in a manageable way. This allows for accurate calculations of reactant amounts, product yields, and other crucial aspects of chemical processes.
Understanding Avogadro's Number
Avogadro's number, denoted as N<sub>A</sub>, is a constant that represents the number of constituent particles (atoms, molecules, ions, or other specified particles) present in one mole of a substance. Its value is approximately 6.022 x 10<sup>23</sup>. This incredibly large number highlights the vastness of the microscopic world at a macroscopic level.
The Historical Context of Avogadro's Number
The concept of Avogadro's number wasn't directly derived from a single experiment. It evolved from the work of several scientists, most notably Amedeo Avogadro, who proposed that equal volumes of gases at the same temperature and pressure contain the same number of molecules. However, the actual determination of Avogadro's number came much later through various experiments, including measurements of the charge of an electron and X-ray diffraction studies of crystals.
The Significance of Avogadro's Number
Avogadro's number is not just a randomly chosen large number; it's a fundamental constant that connects the macroscopic properties of a substance (e.g., mass, volume) to its microscopic properties (e.g., the number of atoms or molecules). This connection is crucial for performing stoichiometric calculations and understanding chemical reactions quantitatively.
The Interplay Between the Mole and Avogadro's Number
The relationship between the mole and Avogadro's number is defined as follows:
1 mole = 6.022 x 10<sup>23</sup> particles
This means that one mole of any substance contains Avogadro's number of particles. This holds true regardless of the type of particle—whether it's an atom of carbon, a molecule of water, or an ion of sodium. This consistency is what makes the mole such a powerful and versatile unit in chemistry.
Examples illustrating the relationship
Let's illustrate this with some examples:
- 1 mole of carbon atoms (C) contains 6.022 x 10<sup>23</sup> carbon atoms.
- 1 mole of water molecules (H<sub>2</sub>O) contains 6.022 x 10<sup>23</sup> water molecules.
- 1 mole of sodium chloride (NaCl) contains 6.022 x 10<sup>23</sup> formula units of NaCl (meaning 6.022 x 10<sup>23</sup> sodium ions and 6.022 x 10<sup>23</sup> chloride ions).
Molar Mass and its Connection to Avogadro's Number
The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). The molar mass is numerically equal to the atomic mass (for elements) or the molecular mass (for compounds) expressed in atomic mass units (amu).
The connection to Avogadro's number lies in the fact that the molar mass represents the mass of Avogadro's number of atoms or molecules. For example, the molar mass of carbon (C) is approximately 12 g/mol. This means that 12 grams of carbon contain 6.022 x 10<sup>23</sup> carbon atoms.
Calculating Moles using Molar Mass
This relationship is extremely useful for converting between mass and the number of moles. We can use the following formula:
Moles (mol) = Mass (g) / Molar Mass (g/mol)
This allows us to determine the number of moles present in a given mass of a substance, and vice versa.
Avogadro's Number and Stoichiometry
Avogadro's number and the concept of the mole are fundamental to stoichiometry, the quantitative study of chemical reactions. Stoichiometric calculations rely heavily on the mole as the unit for quantifying reactants and products. By using moles, we can determine the relative amounts of reactants needed and the amount of products formed in a chemical reaction.
Balancing Chemical Equations and Mole Ratios
Balanced chemical equations show the relative number of moles of reactants and products involved in a reaction. The coefficients in a balanced equation represent the mole ratios. For example, in the reaction:
2H<sub>2</sub> + O<sub>2</sub> → 2H<sub>2</sub>O
The coefficients indicate that 2 moles of hydrogen gas (H<sub>2</sub>) react with 1 mole of oxygen gas (O<sub>2</sub>) to produce 2 moles of water (H<sub>2</sub>O). Using these mole ratios and Avogadro's number, we can calculate the number of molecules involved or the mass of reactants and products.
Beyond Chemistry: Applications of Avogadro's Number
While Avogadro's number is primarily used in chemistry, its implications extend to other scientific fields. It's a fundamental constant that helps us understand the scale of the universe at the atomic and molecular level. Its applications include:
- Material Science: Determining the number of atoms or molecules in a given material sample.
- Physics: Calculations related to atomic structure, nuclear reactions, and radiation.
- Biology: Estimating the number of cells in a biological sample.
- Environmental Science: Analyzing the concentration of pollutants in the environment.
Conclusion
The mole and Avogadro's number are inextricably linked, forming the foundation for quantitative analysis in chemistry and related fields. Understanding their relationship is crucial for anyone studying chemistry, as it allows for the connection between the macroscopic world of measurements and the microscopic world of atoms and molecules. The mole, with its connection to Avogadro's number, provides a practical and manageable way to work with the incredibly large numbers of particles involved in chemical reactions and other scientific phenomena, enabling accurate predictions and understanding of chemical processes. Through this understanding, we can transition smoothly between grams and atoms, bridging the gap between the visible and the invisible worlds of chemistry.
Latest Posts
Latest Posts
-
Name The Four Social Change Theories
Apr 02, 2025
-
Which Of These Infectious Agents Do Not Have Nucleic Acid
Apr 02, 2025
-
How Many Total Atp Are Produced During Glycolysis
Apr 02, 2025
-
Mean And Standard Deviation Of Sampling Distribution Calculator
Apr 02, 2025
-
Dorsal View Of The Sheep Brain
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Relationship Between A Mole And Avogadro's Number . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
