What Is The Shape Of A Gas
Muz Play
Mar 18, 2025 · 6 min read
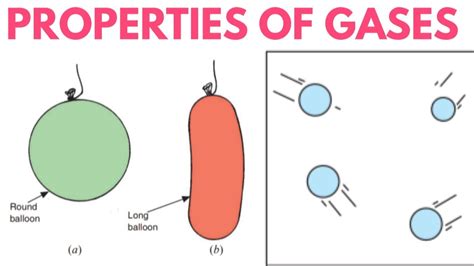
Table of Contents
What is the Shape of a Gas? Exploring the Properties of Gases
The question, "What is the shape of a gas?" might seem deceptively simple. Unlike a solid with its defined structure or a liquid with its surface tension, the shapeless nature of a gas initially leads one to believe it lacks a defined form. However, the reality is more nuanced. Gases, in fact, do have a shape, but understanding this requires exploring their fundamental properties and behavior at a molecular level. This article will delve into the fascinating world of gases, clarifying their shape, exploring their characteristics, and explaining why their seemingly indefinite form is a direct consequence of their unique molecular arrangement.
The Kinetic Molecular Theory: The Foundation of Gas Behavior
To understand the shape of a gas, we need to grasp the kinetic molecular theory (KMT). This theory describes gases as collections of tiny particles (atoms or molecules) in constant, random motion. These particles are incredibly small compared to the distances separating them, and the forces of attraction between them are relatively weak. This weak intermolecular force is the key to understanding why gases don't hold a specific shape.
Key tenets of the Kinetic Molecular Theory:
- Particles are in constant, random motion: Gas particles are in perpetual motion, colliding with each other and the walls of their container. This constant movement is the driving force behind many gas properties.
- Particles are widely separated: The space between gas particles is significantly larger than the particles themselves. This vast space allows gases to be highly compressible.
- Collisions are elastic: When gas particles collide, there's no net loss of kinetic energy. Energy might be transferred between particles, but the total kinetic energy of the system remains constant.
- Particles have negligible volume: The volume of the individual gas particles is considered insignificant compared to the total volume of the gas.
- No intermolecular forces (ideal gas): While real gases experience some intermolecular forces, the ideal gas model assumes these forces are negligible. This simplification makes calculations easier and provides a good approximation of gas behavior under many conditions.
The Shape of a Gas: Container Conformity
So, if gas particles are constantly moving and widely spaced, what determines their shape? The answer is simple: the shape of their container. Gases completely fill any container they occupy. This means a gas takes on the shape of whatever vessel it's confined within, be it a spherical balloon, a cylindrical tank, or a complex-shaped laboratory flask. This adaptability is a direct consequence of the weak intermolecular forces and the significant space between gas particles. There's nothing inherently holding the gas particles in a specific configuration; they simply spread out to occupy the available space.
Illustrative Examples:
- Balloon: Inflate a balloon with air. The air, a mixture of gases, expands to fill the balloon's spherical shape. Puncture the balloon, and the gas disperses, taking on the shape of the surrounding atmosphere.
- Room: The air within a room conforms to the room's shape. The gas molecules move freely, occupying the entire space from floor to ceiling.
- Compressed Gas Cylinder: A compressed gas cylinder holds gas under high pressure. Even though compressed, the gas still conforms to the cylinder's shape, demonstrating that even with closer proximity, the gas still lacks an independent shape.
Factors Influencing Gas Behavior: Pressure, Temperature, and Volume
The behavior of a gas is intricately linked to three key variables: pressure (P), volume (V), and temperature (T). These factors influence the movement and distribution of gas particles, impacting the apparent "shape" indirectly.
Pressure:
Pressure is the force exerted by gas particles per unit area on the walls of their container. Higher pressure means more frequent and forceful collisions between gas particles and the container walls. This doesn't change the gas's tendency to conform to the container's shape, but it does influence the density and the energy of the gas particles.
Temperature:
Temperature reflects the average kinetic energy of the gas particles. Higher temperatures lead to faster-moving particles, resulting in more frequent and energetic collisions. Again, this doesn't alter the gas's shape-adaptability but affects the distribution and kinetic energy of gas molecules within the container.
Volume:
The volume is the space occupied by the gas. Changing the volume changes the available space for the gas particles to move in. Compressing a gas (reducing its volume) increases the pressure and the frequency of particle collisions, while expanding it decreases pressure. The shape of the container still dictates the overall shape of the gas, however, a smaller volume will mean the gas occupies a smaller version of that shape.
Real Gases vs. Ideal Gases: Deviations from the Model
The ideal gas model provides a useful simplification, but real gases deviate from this model, especially at high pressures and low temperatures. At high pressures, the intermolecular forces become more significant, and the volume of the gas particles themselves becomes a more considerable fraction of the total volume. At low temperatures, the kinetic energy of the particles decreases, and intermolecular forces become more influential in determining particle arrangement and behavior. These deviations don't fundamentally change the concept of a gas conforming to its container's shape; however, they introduce complexities and might lead to slight variations from the ideal gas behavior predictions.
Applications and Implications:
Understanding the shape and behavior of gases is crucial in numerous applications:
- Meteorology: Predicting weather patterns relies heavily on understanding atmospheric gas behavior, including pressure, temperature, and volume changes.
- Aerospace Engineering: Designing aircraft and spacecraft requires precise calculations involving the behavior of gases in high-altitude, low-pressure environments.
- Chemical Engineering: Industrial processes involving gas reactions and separations require a thorough understanding of gas properties and thermodynamics.
- Medical Applications: Respiratory systems and anesthesia delivery depend on the properties of respiratory gases (oxygen, nitrogen, carbon dioxide, etc.).
Conclusion: The Adaptable Shape of Gases
In conclusion, while a gas might appear shapeless, it actually conforms perfectly to the shape of its container. This adaptability is a direct consequence of its inherent properties: the constant random motion of its particles, the weak intermolecular forces, and the considerable space between particles. Understanding the kinetic molecular theory and the influence of pressure, temperature, and volume is essential to grasping the true nature of gases and their behavior in various contexts. Although real gases exhibit deviations from ideal behavior, the fundamental principle of shape conformity remains central to understanding their nature. The seemingly simple question of a gas's shape reveals a complex and fascinating world of molecular dynamics and thermodynamic principles.
Latest Posts
Latest Posts
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
-
Delta G Of A Carbonyl Reduction
Mar 18, 2025
-
Difference Between A Strong And Weak Acid
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Shape Of A Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
