What Is The Subscript In Chemistry
Muz Play
Mar 28, 2025 · 6 min read
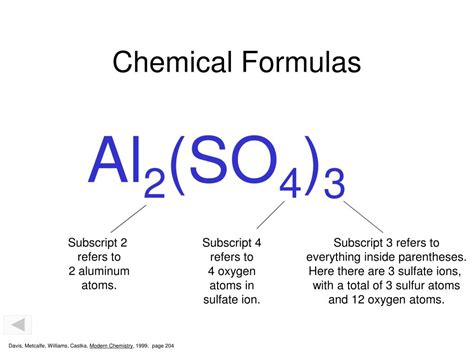
Table of Contents
What is a Subscript in Chemistry? A Comprehensive Guide
Subscripts in chemistry are not just tiny numbers lurking beneath chemical symbols; they are fundamental to understanding the composition and behavior of matter. They hold the key to unlocking stoichiometry, predicting reactions, and comprehending the very building blocks of the universe. This comprehensive guide will delve deep into the world of subscripts, explaining their meaning, function, and significance in various chemical contexts.
Understanding the Basics: What Do Subscripts Represent?
At its core, a subscript in a chemical formula represents the number of atoms of a particular element present in a molecule or compound. It's a crucial piece of information that dictates the ratio of elements within a substance. For example, in the formula for water, H₂O, the subscript '2' indicates that there are two hydrogen atoms for every one oxygen atom in a single molecule of water.
Think of it like a recipe: The subscript acts as the quantity of each ingredient needed to make the final product (the molecule). Without the correct subscripts, the "recipe" would be incorrect, resulting in a different chemical substance entirely.
Subscripts vs. Coefficients: Key Differences
It's important to distinguish subscripts from coefficients, another type of number often found in chemical equations. While both are numerical, their roles are drastically different:
-
Subscripts: Indicate the number of atoms of each element within a single molecule or formula unit. They are part of the chemical formula itself.
-
Coefficients: Indicate the number of molecules or formula units involved in a chemical reaction. They are placed before the chemical formula.
Consider the balanced chemical equation for the combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
- In CH₄ (methane), the subscript '4' indicates four hydrogen atoms per molecule of methane.
- The coefficient '2' before O₂ (oxygen) indicates that two molecules of oxygen are required for the reaction.
Confusing subscripts and coefficients will lead to incorrect interpretations of chemical reactions and compositions.
Subscripts in Different Chemical Contexts
The importance of subscripts extends far beyond simple molecular formulas. Let's explore their role in various chemical scenarios:
1. Ionic Compounds: Representing the Ratio of Ions
Ionic compounds are formed by the electrostatic attraction between positively charged cations and negatively charged anions. Subscripts in ionic formulas represent the ratio of cations to anions needed to achieve electrical neutrality. For example, in sodium chloride (NaCl), the absence of subscripts implies a 1:1 ratio of sodium (Na⁺) and chloride (Cl⁻) ions. In magnesium chloride (MgCl₂), the subscript '2' indicates that two chloride ions are needed to balance the charge of one magnesium ion (Mg²⁺).
Understanding the charges of ions is crucial for correctly writing the subscripts in ionic compounds. This often involves using the criss-cross method to balance the charges and determine the simplest whole-number ratio.
2. Molecular Compounds: Showing the Number of Atoms in a Molecule
Molecular compounds are formed by the sharing of electrons between atoms. Subscripts in molecular formulas directly show the number of atoms of each element present in a single molecule. For example, in carbon dioxide (CO₂), the subscript '2' indicates that each molecule contains two oxygen atoms bonded to a single carbon atom. In glucose (C₆H₁₂O₆), the subscripts show a complex ratio of carbon, hydrogen, and oxygen atoms within the molecule.
3. Hydrates: Indicating the Number of Water Molecules
Hydrates are compounds that incorporate water molecules into their crystal structure. Subscripts in hydrate formulas indicate the number of water molecules associated with each formula unit of the anhydrous (water-free) compound. For example, copper(II) sulfate pentahydrate (CuSO₄·5H₂O) has five water molecules (indicated by the '5' subscript) for every one formula unit of copper(II) sulfate.
4. Polyatomic Ions: Understanding Complex Ions
Polyatomic ions are groups of atoms that carry an overall charge. Subscripts within the formula of a polyatomic ion represent the number of each type of atom within the ion. For example, the sulfate ion (SO₄²⁻) contains one sulfur atom and four oxygen atoms. When using polyatomic ions in formulas for compounds, parentheses are often used to enclose the polyatomic ion, and a subscript is used to indicate the number of polyatomic ions present. For example, in aluminum sulfate, Al₂(SO₄)₃, the subscript '3' shows that three sulfate ions are present per two aluminum ions.
The Importance of Correct Subscripts
Using incorrect subscripts can lead to significant errors in chemistry. A small change in a subscript can result in a completely different compound with drastically different properties. For instance, CO (carbon monoxide) is a toxic gas, while CO₂ (carbon dioxide) is essential for plant life. The difference lies in a single oxygen atom, highlighted by the change in the subscript.
Accurate subscript usage is crucial for:
-
Predicting Chemical Reactions: Correctly written formulas, with accurate subscripts, are essential for balancing chemical equations and accurately predicting the products of a reaction.
-
Understanding Molecular Structure: Subscripts provide direct insight into the number and arrangement of atoms within a molecule, which is fundamental to understanding its properties.
-
Calculating Molar Mass: The molar mass of a compound is directly dependent on the number of atoms of each element present, as indicated by the subscripts in its formula.
-
Stoichiometric Calculations: Subscripts are crucial for performing stoichiometric calculations, determining the amounts of reactants and products involved in a chemical reaction.
Beyond the Basics: Advanced Applications of Subscripts
While the fundamental use of subscripts centers on atom counts, their implications broaden into advanced chemical concepts:
-
Empirical Formulas vs. Molecular Formulas: Empirical formulas represent the simplest whole-number ratio of atoms in a compound, while molecular formulas show the actual number of atoms in a molecule. Subscripts differ between these representations; molecular formulas' subscripts are always a whole-number multiple of empirical formulas' subscripts.
-
Isomers: Isomers are molecules with the same molecular formula (same number and type of atoms, as defined by subscripts) but different structural arrangements. While the subscripts remain the same, the properties of isomers can differ significantly due to their differing structures.
-
Coordination Compounds: In coordination compounds, subscripts inside and outside square brackets indicate the number of ligands (molecules or ions bound to a central metal ion) and the number of complex ions present.
Conclusion: The Unsung Heroes of Chemical Formulas
Subscripts are seemingly simple numerical characters, yet they represent a cornerstone of chemical notation. A deep understanding of their function and significance is crucial for comprehending chemical composition, predicting reactions, and engaging in advanced chemical calculations. Mastering the use of subscripts is vital for any student or professional working in the field of chemistry. Their seemingly small role belies their immense power in unlocking the secrets of the molecular world. From simple water molecules to complex coordination compounds, subscripts provide the essential framework for understanding the world around us at a molecular level. So, next time you encounter a subscript in a chemical formula, remember its power and significance.
Latest Posts
Latest Posts
-
Definition Of Line In A Poem
Mar 31, 2025
-
Speed Of A Wave On A String
Mar 31, 2025
-
Is There Water In The Desert
Mar 31, 2025
-
Definition Of Marginal Analysis In Economics
Mar 31, 2025
-
How To Describe Distribution Of Data
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Subscript In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
