What Is The Substrate Of The Enzyme Amylase
Muz Play
Mar 23, 2025 · 5 min read
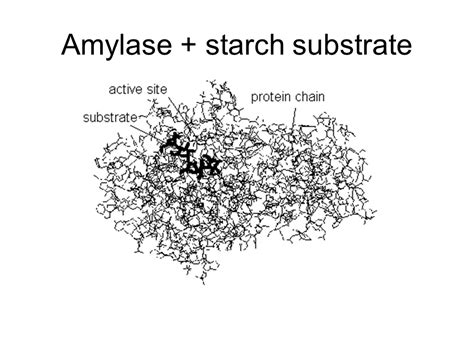
Table of Contents
What is the Substrate of the Enzyme Amylase? A Deep Dive into Amylase Activity and its Significance
Amylase, a ubiquitous enzyme found in various organisms from bacteria to humans, plays a pivotal role in carbohydrate metabolism. Understanding its function requires a thorough grasp of its substrate – the molecule upon which it acts. This article delves into the intricacies of amylase's substrate specificity, the different types of amylases, the mechanisms of their action, and the broader biological significance of amylase activity.
Understanding Amylase: A Primer
Amylases belong to the broader class of hydrolases, enzymes that catalyze the hydrolysis of chemical bonds. More specifically, amylases are glycosidases, meaning they hydrolyze glycosidic bonds – the bonds linking monosaccharides (simple sugars) together in carbohydrates. Their primary substrate is starch, a complex carbohydrate composed of numerous glucose units linked together in various ways. This makes amylase crucial for breaking down starch into simpler, digestible sugars.
Types of Amylase: Alpha, Beta, and Gamma
The world of amylases is not monolithic. Several types exist, each with its own substrate preference and mechanism of action:
-
Alpha-Amylase (α-amylase): This is the most prevalent type of amylase and is found widely distributed in animals, plants, and microorganisms. α-amylase acts randomly along the starch chain, cleaving α-1,4-glycosidic bonds. This leads to the production of shorter oligosaccharides, such as maltose, maltotriose, and dextrins. The action of α-amylase is often described as endo-acting, meaning it cleaves internal bonds within the starch molecule rather than at the ends.
-
Beta-Amylase (β-amylase): β-amylase, in contrast to α-amylase, acts from the non-reducing end of the starch molecule. It cleaves α-1,4-glycosidic bonds, releasing two glucose units at a time in the form of maltose. This makes its action exo-acting. β-amylase is unable to cleave the α-1,6-glycosidic bonds found in branched starch molecules like amylopectin.
-
Gamma-Amylase (γ-amylase): This less common type of amylase is an exo-acting enzyme that hydrolyzes α-1,4-glycosidic bonds from the non-reducing end of starch molecules. However, unlike β-amylase, γ-amylase can also hydrolyze α-1,6-glycosidic bonds, making it capable of acting on branched starch molecules.
Starch: The Primary Substrate of Amylase
Starch, the primary substrate of amylases, is a crucial energy storage polysaccharide in plants. It exists in two major forms:
-
Amylose: A linear polymer consisting of α-1,4-linked glucose units. Amylose forms a helical structure in solution.
-
Amylopectin: A branched polymer of glucose units. It contains primarily α-1,4-linked glucose units, but also features α-1,6-linked branches approximately every 24-30 glucose units. This branching gives amylopectin a more compact structure than amylose.
The specific structure of starch – its degree of branching and chain length – influences the rate and extent of amylase hydrolysis. Amylose, being linear, is generally more susceptible to amylase degradation than amylopectin, due to its simpler structure and lack of branching. The branching in amylopectin can hinder the access of amylases to some of the glycosidic bonds.
The Mechanism of Amylase Action: A Detailed Look
The precise mechanism by which amylases cleave glycosidic bonds involves a complex interplay of factors, including enzyme-substrate binding, catalysis, and product release.
Enzyme-Substrate Binding
The active site of an amylase molecule is specifically shaped to accommodate the starch molecule. This interaction is crucial for orienting the substrate correctly for catalysis. The binding involves various non-covalent interactions, including hydrogen bonds and van der Waals forces, between the enzyme and the glucose units in starch. The precise amino acid residues involved in binding can vary slightly between different types of amylases.
Catalysis: Cleaving the Glycosidic Bond
Once the starch molecule is bound to the active site, the amylase enzyme initiates catalysis. This process typically involves a two-step mechanism:
-
Nucleophilic Attack: A nucleophile (often a water molecule or a catalytic residue on the enzyme) attacks the glycosidic bond, breaking it.
-
Proton Transfer: Protons are transferred between various groups within the active site, stabilizing the transition state and facilitating bond cleavage.
The specific details of the catalytic mechanism can vary depending on the type of amylase. For example, α-amylase utilizes a two-carboxylate mechanism, where two carboxylate groups in the active site act as general acid and base catalysts.
Product Release
After the glycosidic bond is cleaved, the resulting smaller oligosaccharides are released from the active site, allowing the amylase enzyme to bind another starch molecule and repeat the catalytic cycle.
The Significance of Amylase in Biology
Amylase plays a crucial role in various biological processes, including:
-
Digestion: In animals, including humans, amylase is secreted in the saliva (salivary amylase) and pancreas (pancreatic amylase) to break down starch in food. This process releases glucose, providing the body with energy.
-
Plant Metabolism: Plants produce amylases to mobilize stored starch during germination and growth. This process releases glucose, which fuels the development of the seedling.
-
Industrial Applications: Amylases are extensively used in various industrial processes, including:
-
Food Industry: Amylases are used in baking, brewing, and the production of sweeteners.
-
Textile Industry: Amylases are used to desize fabrics.
-
Pharmaceutical Industry: Amylases are used in the production of certain pharmaceuticals.
-
Factors Affecting Amylase Activity
Several factors can influence the activity of amylases:
-
Temperature: Amylases have optimal temperature ranges for activity. High temperatures can denature the enzyme, leading to reduced activity.
-
pH: Amylases also have optimal pH ranges for activity. Extreme pH values can affect the enzyme's structure and function.
-
Substrate Concentration: The rate of amylase activity generally increases with increasing substrate concentration up to a certain point, after which the rate plateaus.
-
Inhibitors: Specific molecules can inhibit amylase activity. These inhibitors can act by competing with the substrate for binding to the active site or by modifying the enzyme's structure.
Conclusion: A Multifaceted Enzyme with Crucial Roles
Amylase, with its diverse isoforms and mechanisms, is an enzyme of significant biological and industrial importance. Its primary substrate, starch, fuels numerous life processes and serves as a raw material for various applications. Understanding the intricacies of amylase activity, its substrate specificity, and its regulation provides valuable insights into carbohydrate metabolism and its role in diverse biological systems. Further research into the structure and function of amylases continues to unveil new details and expand our understanding of this essential enzyme. This deeper comprehension also opens new avenues for technological advancement and applications in diverse fields. The ubiquitous nature of amylase highlights its fundamental role in the life processes of countless organisms, showcasing its evolutionary significance and persistence across the biological spectrum. The continuing investigation into amylases promises further exciting discoveries that will undoubtedly enrich our knowledge of this pivotal enzyme.
Latest Posts
Latest Posts
-
Which Kingdoms Contain Organisms That Are Prokaryotes
Mar 25, 2025
-
What Element Is Found In Proteins
Mar 25, 2025
-
2 Sample Z Test For Proportions
Mar 25, 2025
-
Last Of Five Rhyming Greek Letters
Mar 25, 2025
-
Rusting Of Iron Is Chemical Or Physical Change
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Substrate Of The Enzyme Amylase . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
