What Is The Symbol For Specific Heat
Muz Play
Mar 18, 2025 · 5 min read
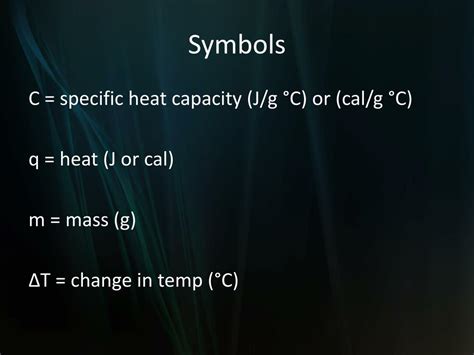
Table of Contents
What is the Symbol for Specific Heat? A Deep Dive into Thermodynamic Properties
Specific heat, a fundamental concept in thermodynamics, describes the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius (or one Kelvin). Understanding its symbol and the nuances surrounding its calculation is crucial in various scientific and engineering fields. This comprehensive article explores the symbol for specific heat, delves into its calculation, discusses its units, and examines its applications.
The Symbol for Specific Heat: c, C<sub>p</sub>, and C<sub>v</sub>
The most common symbol used to represent specific heat is a lowercase c. However, it's important to understand that the specific heat isn't a single, universal constant. It varies depending on several factors, primarily the conditions under which the heat is added. This leads to two crucial distinctions:
1. Specific Heat at Constant Pressure (C<sub>p</sub>):
When heat is added to a substance at constant pressure, its volume may change. This is often the case in everyday scenarios. The specific heat under these conditions is denoted as C<sub>p</sub>. This is because the heat added is partly used to increase the internal energy of the substance and partly to do work against the surrounding pressure as the volume expands.
2. Specific Heat at Constant Volume (C<sub>v</sub>):
If the heating process occurs at constant volume, no work is done against the external pressure because the volume remains unchanged. The specific heat in this case is symbolized as C<sub>v</sub>. All the heat added directly contributes to increasing the internal energy of the substance.
Key Differences and Relationship:
For most substances, C<sub>p</sub> is greater than C<sub>v</sub>. This is because, at constant pressure, some of the added heat is used to perform work (expansion), leaving less energy to raise the temperature. For ideal gases, a simple relationship exists between C<sub>p</sub> and C<sub>v</sub>:
C<sub>p</sub> = C<sub>v</sub> + R
Where R is the ideal gas constant.
Calculating Specific Heat: Methods and Considerations
Calculating specific heat involves measuring the amount of heat transferred (Q) to a known mass (m) of a substance, resulting in a known temperature change (ΔT). The fundamental equation is:
c = Q / (m * ΔT)
However, the practical application often involves more sophisticated techniques and considerations:
1. Calorimetry: A Practical Approach
Calorimetry is a common method for determining specific heat. It involves using a calorimeter, an insulated container designed to minimize heat exchange with the surroundings. A known mass of the substance is heated to a known temperature and then placed in the calorimeter containing a known mass of water at a different temperature. The final equilibrium temperature is measured, and the specific heat can be calculated using the principle of heat exchange:
Q<sub>substance</sub> = -Q<sub>water</sub>
This equation assumes that all the heat lost by the substance is gained by the water (and the calorimeter itself). The specific heat of water is well-known, and the heat gained or lost can be calculated using the formula:
Q = m * c * ΔT
2. Differential Scanning Calorimetry (DSC): A Sophisticated Technique
Differential Scanning Calorimetry (DSC) is a more advanced technique used to determine specific heat over a range of temperatures. It measures the difference in heat flow between a sample and a reference material as they are heated or cooled at a controlled rate. The specific heat can be determined from the heat flow data. DSC is particularly useful for studying phase transitions and other complex thermal behavior.
3. Computational Methods: Molecular Dynamics Simulations
For complex materials or situations where experimental measurements are difficult, computational methods like molecular dynamics simulations can be used to estimate specific heat. These simulations model the interactions between atoms or molecules and can predict thermodynamic properties like specific heat.
Units of Specific Heat
The units of specific heat depend on the units used for heat, mass, and temperature. Commonly used units include:
- J/(kg·K): Joules per kilogram-Kelvin (SI unit)
- J/(g·°C): Joules per gram-degree Celsius
- cal/(g·°C): Calories per gram-degree Celsius
- Btu/(lb·°F): British thermal units per pound-degree Fahrenheit
Factors Affecting Specific Heat
Several factors influence the specific heat of a substance:
- Temperature: Specific heat is often temperature-dependent, meaning it varies with temperature.
- Pressure: As discussed earlier, pressure significantly impacts specific heat, leading to the distinction between C<sub>p</sub> and C<sub>v</sub>.
- Phase: The specific heat of a substance differs in its solid, liquid, and gaseous phases. Phase transitions (melting, boiling) involve significant heat absorption without a temperature change.
- Chemical Composition: The chemical composition of a substance strongly affects its specific heat. Different materials have different molecular structures and intermolecular forces, leading to variations in their specific heat.
Applications of Specific Heat
Specific heat is a crucial parameter in various scientific and engineering applications:
- Material Science: Determining the specific heat of new materials is essential for their characterization and selection for specific applications.
- Chemical Engineering: Specific heat is used in designing and optimizing chemical processes involving heat transfer.
- Meteorology: Understanding the specific heat of air and water is important for weather prediction and climate modeling.
- Thermodynamics: Specific heat is a fundamental property used in numerous thermodynamic calculations and analyses.
- Energy Storage: Materials with high specific heat are attractive for thermal energy storage applications.
Conclusion: Beyond the Symbol
While the symbol c (or C<sub>p</sub> and C<sub>v</sub>) serves as a concise representation of specific heat, a true understanding requires grasping the underlying concepts and its dependence on various factors. The ability to calculate and interpret specific heat data is critical for addressing various challenges in science and engineering. This article has aimed to provide a comprehensive overview, equipping you with the knowledge to navigate the intricacies of this fundamental thermodynamic property. Remember to always consider the conditions (constant pressure or constant volume) when dealing with specific heat values to ensure accuracy in your calculations and applications. The use of appropriate units and the understanding of the experimental and computational methods employed in determining specific heat are also crucial elements for a thorough grasp of this important concept. By understanding these intricacies, you can leverage the power of specific heat in various fields and contribute to advancements in diverse areas of research and technology.
Latest Posts
Latest Posts
-
Is Water A Mixture Or Pure Substance
Mar 18, 2025
-
Which Base Is Not Found In Rna
Mar 18, 2025
-
So Long To Pinky Here Comes The Thumb
Mar 18, 2025
-
When Two Amino Acids Are Joined Together
Mar 18, 2025
-
What Is The Difference Between Chemical Reaction And Nuclear Reaction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Symbol For Specific Heat . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
