What Temperature Does Water Freeze At In Kelvin
Muz Play
Mar 18, 2025 · 6 min read
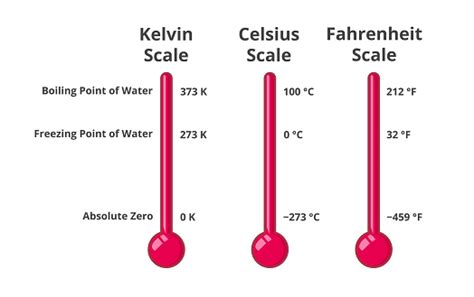
Table of Contents
What Temperature Does Water Freeze at in Kelvin?
The freezing point of water, a seemingly simple concept, holds significant importance across various scientific disciplines and everyday life. While we commonly express this temperature in Celsius (°C) or Fahrenheit (°F), understanding it in Kelvin (K) provides a deeper insight into the fundamental nature of temperature and its impact on matter. This article will delve into the intricacies of water's freezing point in Kelvin, exploring its scientific basis, practical applications, and the nuances that might affect this crucial value.
Understanding the Kelvin Scale
Before diving into the freezing point of water, let's establish a firm understanding of the Kelvin scale itself. Unlike Celsius and Fahrenheit, which are relative scales with arbitrary zero points, Kelvin is an absolute temperature scale. Its zero point, 0 Kelvin (also known as absolute zero), represents the theoretical absence of all thermal energy. At absolute zero, all molecular motion ceases. This absolute scale makes Kelvin particularly valuable in scientific calculations and understanding thermodynamic principles.
The relationship between Kelvin and Celsius is straightforward:
- K = °C + 273.15
This equation allows for easy conversion between the two scales.
The Freezing Point of Water in Kelvin: The Answer
The freezing point of pure water at standard atmospheric pressure (1 atmosphere or 101.325 kPa) is 273.15 Kelvin. This is equivalent to 0°C or 32°F. It's crucial to highlight the phrase "pure water" and "standard atmospheric pressure." These factors can significantly influence the precise freezing point.
The Significance of 273.15 K
This specific value, 273.15 K, isn't just a number; it's a fundamental constant in physics and chemistry. It serves as a benchmark for many calculations and experiments, forming the basis for understanding phase transitions, thermodynamics, and the behavior of matter at different temperatures.
Factors Affecting the Freezing Point of Water
While 273.15 K is the standard freezing point, several factors can cause deviations from this value:
1. Pressure:
Increasing pressure lowers the freezing point of water. This is a unique property of water, unlike most substances where increased pressure raises the freezing point. This anomaly is due to the unusual structure of ice, which is less dense than liquid water. At high pressures, the liquid phase becomes more stable than the solid phase, leading to a lower freezing temperature. This effect is relatively small at pressures near standard atmospheric pressure but becomes more pronounced at significantly higher pressures.
2. Impurities:
Dissolved substances in water, such as salts or other solutes, lower its freezing point. This is known as freezing point depression. The extent of the depression depends on the concentration of the solute. This principle is utilized in various applications, such as de-icing roads in winter. Adding salt to water lowers its freezing point, preventing ice formation at temperatures slightly below 0°C.
3. Isotopic Composition:
Water molecules are composed of hydrogen and oxygen atoms. However, hydrogen has two stable isotopes: protium (¹H) and deuterium (²H). Water containing heavier isotopes of hydrogen (deuterium oxide or heavy water) freezes at a slightly higher temperature than regular water. This difference is relatively small but measurable and relevant in certain scientific studies.
4. Supercooling:
Under specific conditions, water can be cooled below its freezing point without actually freezing. This phenomenon is called supercooling. Supercooled water is metastable, meaning it's in a state of unstable equilibrium. A slight disturbance, such as a vibration or the introduction of a nucleation site (a small particle around which ice crystals can form), will cause it to rapidly freeze.
Practical Applications of Water's Freezing Point
The freezing point of water is not just a theoretical value; it has numerous practical implications in various fields:
1. Food Preservation:
Freezing is a common method of food preservation. The process relies on lowering the temperature of food below its freezing point, inhibiting the growth of microorganisms and slowing down enzymatic reactions that cause spoilage. Understanding the freezing point and the factors that can affect it is crucial in optimizing food preservation techniques.
2. Ice Formation in Nature:
The freezing point of water is pivotal in understanding natural phenomena like the formation of ice in lakes, rivers, and glaciers. The temperature of water bodies, along with the factors discussed earlier, determines when and how ice forms, impacting various ecosystems and weather patterns.
3. Industrial Processes:
Many industrial processes involve the freezing or thawing of water or water-based solutions. Understanding the precise freezing point under specific conditions is crucial in controlling these processes and ensuring efficiency and safety.
4. Cryopreservation:
Cryopreservation, the process of preserving biological materials at very low temperatures, relies on the principles of freezing and thawing. Precise control over freezing rates and temperature is critical in preventing damage to the preserved materials.
Beyond the Basics: Exploring Advanced Concepts
The freezing point of water, while seemingly simple, opens doors to a complex world of scientific inquiry. Let's explore some advanced concepts that build upon our understanding:
1. Phase Diagrams:
Phase diagrams visually represent the phases of a substance (solid, liquid, gas) as a function of temperature and pressure. The phase diagram of water shows the relationship between its solid, liquid, and gaseous phases, illustrating how the freezing point varies with pressure.
2. Thermodynamics of Freezing:
The freezing of water is a thermodynamic process governed by the principles of enthalpy (heat content) and entropy (disorder). The freezing process involves the release of heat (latent heat of fusion) as water molecules transition from a disordered liquid state to a more ordered solid state.
3. Nucleation and Crystal Growth:
The formation of ice crystals involves two key processes: nucleation (the formation of initial ice nuclei) and crystal growth (the subsequent expansion of these nuclei into larger ice crystals). Understanding these processes is critical in controlling ice formation in various applications, such as in food processing and cryopreservation.
4. Water's Anomalous Properties:
Water possesses several anomalous properties, including its lower density in solid form compared to liquid form. This anomaly is linked to the hydrogen bonding network in water molecules, which leads to the unique properties of ice and its lower freezing point compared to similar compounds.
Conclusion: The Enduring Importance of 273.15 K
The freezing point of water at 273.15 Kelvin is more than just a numerical value; it represents a fundamental constant with far-reaching implications across scientific disciplines and everyday life. Understanding this value, along with the factors that can influence it, is essential for comprehending various natural phenomena, optimizing industrial processes, and developing advanced technologies. Further research continues to unravel the complexities surrounding water's behavior at its freezing point and beyond, expanding our knowledge of this essential substance. The seemingly simple question of what temperature water freezes at in Kelvin opens up a fascinating exploration into the intricacies of physics, chemistry, and the world around us.
Latest Posts
Latest Posts
-
Electric Potential At A Point Due To A Point Charge
Mar 18, 2025
-
Science Terms That Start With J
Mar 18, 2025
-
What Is The Unit Of Atomic Radius
Mar 18, 2025
-
Which Type Of Hormone Is Lipid Soluble
Mar 18, 2025
-
How To Find Instantaneous Rate Of Reaction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Temperature Does Water Freeze At In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
