When Bonds Are Broken Energy Is
Muz Play
Mar 21, 2025 · 6 min read
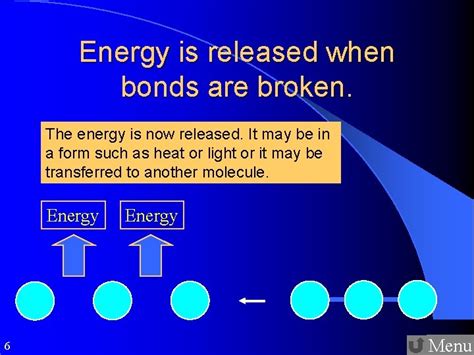
Table of Contents
When Bonds Are Broken, Energy Is… Released: A Deep Dive into Chemical Bonds and Thermodynamics
Chemical reactions are the bedrock of all processes in the universe, from the smallest cellular activities to the largest stellar explosions. At the heart of these reactions lies the breaking and forming of chemical bonds, processes intrinsically linked to energy transfer. When bonds are broken, energy is released – or absorbed – a fundamental principle governing chemical thermodynamics. This article delves deep into this principle, exploring the various types of chemical bonds, the energetic consequences of bond breakage, and the implications for various scientific fields.
Understanding Chemical Bonds: The Glue of Molecules
Before exploring the energetics of bond breaking, we need a solid understanding of what chemical bonds actually are. These are the forces that hold atoms together in molecules and compounds. Several types of bonds exist, each with its unique characteristics and associated energy levels.
Covalent Bonds: Sharing is Caring (and Energetically Favorable)
Covalent bonds form when atoms share electrons to achieve a more stable electron configuration, usually resembling a noble gas. The strength of a covalent bond depends on factors like the electronegativity difference between the atoms involved and the number of shared electron pairs. A stronger bond requires more energy to break, while a weaker bond requires less. Examples of molecules held together by covalent bonds include water (H₂O), methane (CH₄), and glucose (C₆H₁₂O₆). Breaking a covalent bond always requires energy input. This energy is often supplied in the form of heat or light.
Ionic Bonds: Opposites Attract (and Electrostatically Bind)
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. One atom loses electrons (becoming a positively charged cation), and another atom gains those electrons (becoming a negatively charged anion). The strong electrostatic forces holding these ions together constitute the ionic bond. Table salt (NaCl) is a classic example of an ionic compound. While forming an ionic bond releases energy, breaking it requires energy input. The energy required is related to the magnitude of the electrostatic forces.
Hydrogen Bonds: Weak but Vital Interactions
Hydrogen bonds are a special type of dipole-dipole interaction that occurs when a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen or nitrogen) is attracted to another electronegative atom in a different molecule. These bonds are significantly weaker than covalent or ionic bonds but play crucial roles in biological systems, influencing the structure and properties of proteins, DNA, and water. Breaking a hydrogen bond requires relatively less energy compared to covalent or ionic bonds.
Van der Waals Forces: Weak, Transient Interactions
Van der Waals forces are the weakest type of intermolecular forces. They arise from temporary fluctuations in electron distribution around atoms or molecules, creating transient dipoles that attract each other. While individually weak, these forces become significant when many molecules interact, contributing to properties like boiling points and melting points. The energy required to break Van der Waals forces is minimal.
The Energetics of Bond Breaking: Enthalpy and Bond Energy
The energy change associated with breaking a chemical bond is quantified by the bond dissociation energy (BDE) or bond enthalpy. This value represents the energy required to break one mole of a specific type of bond in the gaseous phase. Bond energies are usually expressed in kilojoules per mole (kJ/mol). Higher bond energies indicate stronger bonds.
Exothermic vs. Endothermic Reactions: Energy Release and Absorption
When bonds are broken, the overall energy change depends on the relative strengths of the bonds broken and the bonds formed. If the energy released by forming new bonds is greater than the energy required to break the existing bonds, the reaction is exothermic, and energy is released to the surroundings. This is typically observed as heat. Conversely, if the energy required to break bonds exceeds the energy released by forming new bonds, the reaction is endothermic, and energy is absorbed from the surroundings.
Hess's Law: Calculating Enthalpy Changes
Hess's Law states that the enthalpy change for a reaction is independent of the pathway taken. This means that the total enthalpy change for a reaction can be calculated by summing the enthalpy changes of individual steps in the reaction, including bond breaking and bond formation. This law is particularly useful for complex reactions where directly measuring the overall enthalpy change is difficult.
Applications and Implications Across Scientific Fields
The principle that when bonds are broken, energy is either released or absorbed, has profound implications across various scientific disciplines.
Chemistry: Understanding Reaction Mechanisms and Kinetics
Understanding bond energies is essential for predicting the feasibility and kinetics of chemical reactions. Reactions involving the breaking of weak bonds tend to proceed faster than those involving strong bonds. By analyzing bond energies, chemists can determine the rate-limiting steps in a reaction and design catalysts to increase reaction rates.
Biochemistry: Metabolism and Energy Production
Metabolic processes, the chemical reactions sustaining life, heavily rely on bond breaking and formation. Cellular respiration, for example, involves the breaking of glucose bonds, releasing energy stored within those bonds to produce ATP, the cell's primary energy currency. Enzymes, biological catalysts, facilitate these bond-breaking and bond-forming processes, lowering the activation energy required.
Materials Science: Designing New Materials with Desired Properties
The properties of materials, such as strength, hardness, and melting point, are directly related to the types and strengths of the bonds holding the constituent atoms or molecules together. Materials scientists utilize their understanding of bond energies to design new materials with specific properties. For instance, designing strong and lightweight materials for aerospace applications requires an in-depth understanding of the underlying chemical bonds.
Astrophysics: Stellar Nucleosynthesis and Energy Production
The immense energy production in stars stems from nuclear fusion reactions, where atomic nuclei fuse together, forming new, heavier nuclei. These fusion reactions involve breaking and forming extremely strong nuclear bonds, releasing vast amounts of energy. Understanding this process is fundamental to astrophysics, aiding in models of stellar evolution and energy generation.
Conclusion: A Fundamental Principle with Broad Applications
The concept that when bonds are broken, energy is released or absorbed, forms the cornerstone of numerous scientific disciplines. Whether studying simple chemical reactions or complex biological processes, or exploring the vast cosmos, understanding the energetics of bond breaking provides crucial insight into the mechanisms driving these processes. The quantification of bond energies and enthalpy changes using techniques like Hess's Law provides a powerful tool for predicting reaction outcomes, designing new materials, and understanding fundamental processes in nature. From the mundane to the magnificent, the energetic consequences of bond breakage are fundamental to understanding the universe around us.
Latest Posts
Latest Posts
-
What Are The Possible Genotypes Of The Offspring
Mar 28, 2025
-
What Is The Unit Of Measure For Kinetic Energy
Mar 28, 2025
-
How To Solve The System Of Equations Algebraically
Mar 28, 2025
-
How Many Hydrogen Bonds Are Found Between C G
Mar 28, 2025
-
What Is The Simplest Form Of A Radical Expression
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about When Bonds Are Broken Energy Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
