How Many Hydrogen Bonds Are Found Between C-g
Muz Play
Mar 28, 2025 · 6 min read
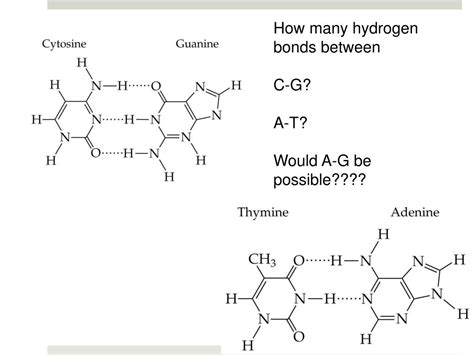
Table of Contents
How Many Hydrogen Bonds are Found Between C-G Base Pairs?
Understanding the intricacies of DNA structure is fundamental to grasping the mechanisms of life itself. Central to this understanding is the nature of the hydrogen bonds that hold the DNA double helix together. While the overall structure is elegantly simple, the details of base pairing, specifically the number of hydrogen bonds between different nucleotide bases, are crucial. This article delves deep into the question: how many hydrogen bonds are found between cytosine (C) and guanine (G) base pairs? We'll explore the chemistry behind these bonds, their significance in DNA stability, and the broader implications for genetics and biotechnology.
The Foundation: Understanding Hydrogen Bonds
Before diving into the specifics of C-G base pairing, let's establish a firm understanding of hydrogen bonds themselves. These are a special type of dipole-dipole attraction between molecules, not a true chemical bond like covalent bonds. They occur when a hydrogen atom bonded to a highly electronegative atom (like oxygen or nitrogen) is attracted to another electronegative atom in a different molecule. This attraction arises from the partial positive charge on the hydrogen atom (δ+) and the partial negative charge on the electronegative atom (δ-).
Hydrogen bonds are relatively weak compared to covalent bonds, but their collective strength is significant in biological systems. The cumulative effect of numerous hydrogen bonds provides the structural integrity needed for complex molecules like DNA and proteins. The strength of a hydrogen bond depends on several factors, including the electronegativity of the atoms involved, the distance between them, and the surrounding environment.
Cytosine (C) and Guanine (G): A Perfect Pairing
Cytosine (C) and guanine (G) are two of the four nitrogenous bases that make up the building blocks of DNA. They are purine and pyrimidine bases, respectively, meaning they have different ring structures. This difference is crucial for their specific pairing.
Cytosine possesses an amino group (-NH2) and a carbonyl group (=O). Guanine, on the other hand, has an amino group (-NH2) and a carbonyl group (=O), as well as an additional amino group. It's the precise arrangement of these functional groups that allows for the formation of hydrogen bonds between C and G.
The Hydrogen Bond Count: Three Strong Bonds
The answer to the central question is: three hydrogen bonds are found between a cytosine (C) and a guanine (G) base pair in DNA. This is a key difference from the adenine (A) – thymine (T) base pair, which is held together by only two hydrogen bonds.
Let's break down the three hydrogen bonds:
-
Hydrogen bond 1: A hydrogen atom from the amino group (-NH2) of cytosine forms a hydrogen bond with the carbonyl oxygen (=O) of guanine.
-
Hydrogen bond 2: A hydrogen atom from the amino group (-NH2) of guanine forms a hydrogen bond with a nitrogen atom in the ring structure of cytosine.
-
Hydrogen bond 3: A hydrogen atom from the imino group (=NH) of guanine forms a hydrogen bond with the carbonyl oxygen (=O) of cytosine.
The formation of these three hydrogen bonds creates a highly stable and specific pairing between C and G. This specificity is critical for the accurate replication and transcription of genetic information.
The Significance of Three Hydrogen Bonds: Stability and Function
The presence of three hydrogen bonds between C and G has profound implications for DNA structure and function:
-
Increased Stability: The three hydrogen bonds contribute to the overall stability of the DNA double helix. The stronger C-G base pairing compared to A-T pairing enhances the resistance of DNA to denaturation (separation of the strands), particularly at higher temperatures or under extreme pH conditions. Regions of DNA with a higher G-C content tend to be more thermally stable.
-
Precise Replication: The specificity of C-G base pairing ensures accurate replication of the DNA molecule during cell division. Each base pairs with its complementary base, ensuring that the genetic information is faithfully passed on to daughter cells. Errors in base pairing are minimized due to the strength and specificity of these hydrogen bonds.
-
Regulation of Gene Expression: The GC content of a DNA region can influence gene expression. Regions with high GC content are often associated with tightly packed chromatin structures, leading to reduced gene transcription. Conversely, regions with lower GC content may have more open chromatin structures, allowing for easier access of transcriptional machinery.
-
DNA Structure and Shape: The number of hydrogen bonds influences the overall shape and structure of the DNA molecule. The stronger interaction between C-G pairs can slightly alter the helical twist and groove dimensions of the DNA double helix.
-
Applications in Biotechnology: The knowledge of hydrogen bonding in DNA is exploited in various biotechnological applications. Techniques like polymerase chain reaction (PCR) rely on the controlled denaturation and renaturation of DNA, processes that are directly influenced by the number and strength of hydrogen bonds.
Beyond the Basics: Factors Influencing Hydrogen Bond Strength
While we've established that three hydrogen bonds connect C and G, it's important to acknowledge that the strength of these bonds isn't static. Several factors can modulate their strength:
-
Base Stacking Interactions: In addition to hydrogen bonding, the stacking interactions between the aromatic bases contribute significantly to the overall stability of DNA. These hydrophobic interactions occur between adjacent base pairs, further stabilizing the double helix.
-
Solvent Effects: The surrounding aqueous environment significantly influences hydrogen bond strength. Water molecules can compete for hydrogen bonding partners, potentially weakening the bonds between base pairs.
-
Ionic Strength: The presence of ions in the solution can also affect hydrogen bond strength by shielding the charges on the participating atoms.
-
Temperature: Increasing temperature weakens hydrogen bonds, leading to DNA denaturation. This is exploited in many laboratory techniques.
-
pH: Changes in pH can alter the protonation state of the bases, impacting hydrogen bond formation.
Variations and Exceptions: Considering the Broader Context
While the standard base pairing shows three hydrogen bonds between C and G, it's crucial to consider that exceptions and variations exist. These usually occur under non-physiological conditions or in specific contexts:
-
Mutations: Mutations can alter the base sequence, potentially disrupting the normal hydrogen bonding pattern. These mutations can lead to various genetic disorders.
-
Non-canonical Base Pairs: Under certain circumstances, non-canonical base pairs might form, involving slightly different hydrogen bonding interactions. These are often seen in unusual DNA structures or in specific protein-DNA complexes.
-
Modified Bases: The presence of modified bases in DNA (e.g., methylated cytosine) can influence hydrogen bonding interactions.
Conclusion: The Importance of C-G Base Pairing
The fact that three hydrogen bonds are found between cytosine and guanine base pairs is a cornerstone of molecular biology. This seemingly simple detail underpins numerous critical processes, from DNA replication and transcription to the overall stability and three-dimensional structure of the DNA double helix. Understanding the nuances of these hydrogen bonds is essential for advancing our knowledge of genetics, genomics, and biotechnology. Further research continues to unravel the complexities of DNA interactions, revealing ever-more intricate details about this fundamental molecule of life. The stability afforded by these three hydrogen bonds is vital for the integrity of our genetic code and the continuation of life as we know it.
Latest Posts
Latest Posts
-
Which Statement Describes The Citric Acid Cycle
Mar 31, 2025
-
Why Do Plants Love Water In Bio Terms
Mar 31, 2025
-
Identifying The Important Intermolecular Forces In Pure Compounds
Mar 31, 2025
-
Why Does Km Increase In Competitive Inhibition
Mar 31, 2025
-
What Is The Electron Configuration Of Beryllium
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Hydrogen Bonds Are Found Between C-g . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
