Where Is The Most Mass In An Atom
Muz Play
Mar 18, 2025 · 5 min read
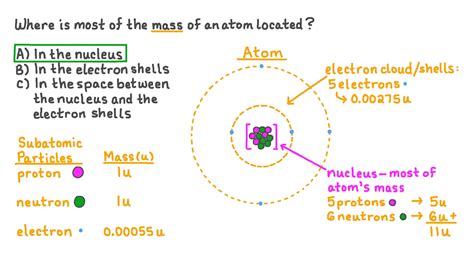
Table of Contents
Where is the Most Mass in an Atom? Delving into the Nucleus
The atom, the fundamental building block of matter, is a fascinating realm of physics. Understanding its structure is key to grasping the properties of everything around us. A common question that arises when studying atoms is: where is the most mass concentrated? The short answer is: the nucleus. But let's delve deeper into the specifics, exploring the components of an atom and their relative contributions to its overall mass.
The Atomic Structure: A Brief Overview
An atom consists of three primary subatomic particles:
- Protons: Positively charged particles residing within the atom's nucleus.
- Neutrons: Neutral particles (no charge) also located in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
The nucleus, a tiny, dense region at the atom's center, houses both protons and neutrons. The electrons, on the other hand, occupy a much larger volume surrounding the nucleus, but their contribution to the atom's overall mass is negligible compared to the nucleus.
Mass of Subatomic Particles: A Comparative Analysis
To understand where the majority of an atom's mass resides, we need to compare the masses of protons, neutrons, and electrons. While precise measurements exist, we can use approximate values to illustrate the point:
- Proton Mass: Approximately 1 atomic mass unit (amu)
- Neutron Mass: Approximately 1 amu
- Electron Mass: Approximately 0.0005 amu
Notice the stark difference! The mass of an electron is almost 2000 times smaller than that of a proton or neutron. This significant disparity clearly indicates that the protons and neutrons contribute almost the entirety of the atom's mass.
The Significance of the Atomic Mass Unit (amu)
The atomic mass unit (amu), also known as the dalton (Da), is a standard unit for expressing the mass of atoms and molecules. It's defined as one-twelfth the mass of a carbon-12 atom. Using this unit allows for convenient comparison of the masses of different subatomic particles and atoms.
The Nucleus: The Mass Heavyweight Champion
The combined mass of the protons and neutrons in the nucleus overwhelmingly dominates the atom's total mass. Electrons, while crucial for chemical interactions and determining an atom's charge, contribute so little to the overall mass that they are often ignored in mass calculations.
This concentration of mass in the nucleus has profound implications:
- Density: The nucleus boasts an incredibly high density, far surpassing the density of any known material.
- Nuclear Reactions: The immense forces within the nucleus are responsible for nuclear reactions, such as fission and fusion, which release tremendous amounts of energy.
- Atomic Stability: The ratio of protons to neutrons within the nucleus determines an atom's stability. Unstable isotopes undergo radioactive decay, emitting particles and energy to achieve a more stable configuration.
Isotopes and Mass Number
Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. This variation in neutron number leads to different mass numbers. The mass number is the total number of protons and neutrons in an atom's nucleus. Since protons and neutrons contribute almost equally to the mass, the mass number provides a good approximation of an atom's mass.
Beyond the Basics: Exploring Nuclear Forces
The incredibly strong forces holding the protons and neutrons together within the nucleus are known as nuclear forces. These forces are significantly stronger than the electromagnetic forces that repel the positively charged protons. Without these strong nuclear forces, the nucleus would simply fly apart due to electrostatic repulsion.
Understanding these nuclear forces is crucial for comprehending:
- Nuclear Stability: The balance between the strong nuclear forces and the electromagnetic repulsion determines an atom's stability and its tendency to undergo radioactive decay.
- Nuclear Reactions: The energy released during nuclear reactions, like fission and fusion, originates from changes in the strong nuclear forces within the nucleus.
- Nuclear Physics: The study of nuclear physics delves into the intricacies of the nucleus, its composition, and the forces governing its behavior.
Practical Applications: Mass Spectrometry and its Uses
Mass spectrometry is a powerful analytical technique used to measure the mass-to-charge ratio of ions. It's employed in various fields, providing valuable information about the composition of different substances.
In the context of determining where the most mass resides in an atom, mass spectrometry provides direct evidence:
- Isotope Analysis: Mass spectrometry allows scientists to precisely determine the relative abundance of different isotopes within a sample. This data further reinforces the understanding of the significant contribution of protons and neutrons to an atom's mass.
- Molecular Weight Determination: By analyzing the mass-to-charge ratios of ionized molecules, mass spectrometry helps determine the molecular weight of complex compounds. This information is essential in various fields, including biochemistry, pharmaceuticals, and environmental science.
- Forensic Science: Mass spectrometry plays a crucial role in forensic science, assisting in the identification of drugs, explosives, and other materials relevant to criminal investigations.
Conclusion: The Nucleus Reigns Supreme
In conclusion, the overwhelming majority of an atom's mass is concentrated within its nucleus. The protons and neutrons, each contributing approximately 1 amu, far outweigh the negligible mass of the orbiting electrons. This concentration of mass in the nucleus is responsible for many of the atom's properties, from its density to its potential for nuclear reactions. Understanding this fundamental aspect of atomic structure is essential for comprehending the behavior of matter and its applications across numerous scientific disciplines. The study of the nucleus and its components continues to be a vibrant area of research, pushing the boundaries of our knowledge about the universe and its fundamental building blocks. Further advancements in techniques like mass spectrometry promise even deeper insights into the fascinating world of atoms and their nuclei. The nucleus, therefore, rightfully claims the title of the atom's mass heavyweight champion.
Latest Posts
Latest Posts
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
-
Delta G Of A Carbonyl Reduction
Mar 18, 2025
-
Difference Between A Strong And Weak Acid
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Where Is The Most Mass In An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
