Where On The Periodic Table Are Metals Found
Muz Play
Mar 29, 2025 · 5 min read
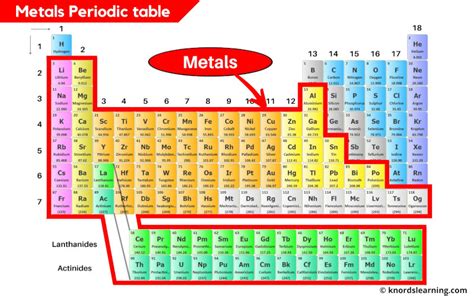
Table of Contents
Where on the Periodic Table are Metals Found? A Comprehensive Guide
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. One of the most fundamental classifications within the table is the distinction between metals, nonmetals, and metalloids. This article delves deep into the location of metals on the periodic table, exploring their characteristics, groupings, and exceptions. Understanding this spatial distribution is crucial for grasping the chemical behavior and applications of these essential materials.
The Broad Sweep: Metals Dominate the Left
The vast majority of metals reside on the left and center of the periodic table. This isn't a perfectly defined line, but a general trend that's readily apparent. As you move from left to right across a period (a horizontal row), you transition from predominantly metallic elements to nonmetals. This gradual shift is reflected in the changing properties of the elements.
The Alkali Metals (Group 1): Highly Reactive Metals
The alkali metals, found in Group 1 (the first column), are the most reactive metals. This high reactivity stems from their single valence electron, which they readily lose to form +1 ions. They are all soft, silvery-white metals with low melting points. Their extreme reactivity means they are never found in their pure elemental form in nature – they are always combined with other elements, typically in compounds. Examples include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
The Alkaline Earth Metals (Group 2): Less Reactive, but Still Metallic
Group 2 houses the alkaline earth metals, which are less reactive than the alkali metals, possessing two valence electrons. They are also silvery-white metals, but generally harder and denser than their Group 1 counterparts. Again, they are too reactive to exist freely in nature and are found in various minerals. Examples include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
The Transition Metals (Groups 3-12): A Diverse Group with Variable Properties
Occupying the central block of the periodic table are the transition metals. This extensive group (Groups 3-12) exhibits a wide range of properties and oxidation states, giving rise to their diverse applications. They are generally hard, dense, and have high melting points. Many are excellent conductors of heat and electricity, making them vital in various technological applications. Iron (Fe), copper (Cu), gold (Au), silver (Ag), and platinum (Pt) are just a few examples of the numerous transition metals. The d-block electrons, which are involved in bonding, explain their variable oxidation states and rich chemistry.
The Post-Transition Metals (Groups 13-15): Bridging the Gap
The elements in Groups 13-15 showcase a transition from metallic to non-metallic character. The elements at the beginning of these groups exhibit metallic properties, though these properties are less pronounced than those of the alkali, alkaline earth, and transition metals. These elements, often referred to as post-transition metals, display a blend of metallic and non-metallic characteristics. Aluminum (Al), tin (Sn), and lead (Pb) exemplify this intermediary behavior. Their properties reflect this transitional position, with increasing electronegativity and a tendency to form covalent bonds as you progress across the periods.
The Lanthanides and Actinides: Two Special Rows
Located separately at the bottom of the periodic table are the lanthanides (rare earth elements) and the actinides. These elements are f-block elements, meaning their outermost electrons are found in the f-orbital. Both series are primarily metallic, exhibiting similar chemical properties within their respective series. The lanthanides find applications in various high-tech materials, while the actinides are predominantly radioactive elements.
The Fuzzy Boundaries: Metalloids and the Metal/Nonmetal Border
The demarcation between metals and nonmetals isn't a sharp line. There's a region where elements exhibit properties intermediate between typical metals and nonmetals. These elements are called metalloids, also known as semimetals.
Where are the Metalloids?
Metalloids are strategically positioned along a diagonal staircase-like line separating metals from nonmetals. This line generally runs from boron (B) to astatine (At). Elements along this line exhibit properties that are a blend of both metals and nonmetals, depending on the conditions. Their conductivity is often sensitive to temperature, pressure, or the presence of impurities. Silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), and tellurium (Te) are prime examples of metalloids. Their unique properties make them essential in semiconductors and other electronic components.
Exceptions and Anomalies: Hydrogen and Others
While the vast majority of metals are easily identifiable based on their position on the periodic table, there are a few exceptions. Hydrogen (H), situated in Group 1, is typically considered a nonmetal due to its relatively high electronegativity and its tendency to form covalent bonds. However, under high pressure, it can exhibit metallic behavior.
Certain other elements, depending on the specific circumstances, can display properties that blur the lines of traditional metal classifications. The exact behavior of an element can depend on the specific conditions and how it interacts within compounds.
The Importance of Understanding Metal Location
Knowing where metals are located on the periodic table is essential for several reasons:
- Predicting Properties: The periodic table allows for the prediction of chemical and physical properties. Metals generally exhibit similar traits within their groups and across periods.
- Understanding Reactivity: The position of a metal on the table can help predict its reactivity, crucial in chemical reactions and material science.
- Designing Materials: This understanding is vital in materials science for designing alloys and new materials with specific desired properties.
- Technological Applications: The unique properties of metals, predictable based on their location, drive the development and application in numerous technologies.
Conclusion: A Spatial Map to Metallic Behavior
The periodic table provides a concise yet comprehensive map for understanding the properties of elements. The vast majority of metals are found on the left and center, with a clear trend of decreasing metallic character as one moves towards the right side of the table. Understanding this spatial distribution, including the transitional metalloids and exceptions, is fundamental for chemists, materials scientists, and anyone interested in the behavior and applications of metals. The periodic table isn't just a chart; it's a powerful tool for unlocking the secrets of the elements and harnessing their unique capabilities. By mastering the location and associated properties of metals on the table, we unlock a pathway to innovative materials and technologies.
Latest Posts
Latest Posts
-
What Is A Row In The Periodic Table
Mar 31, 2025
-
Principle Of Conservation Of Angular Momentum
Mar 31, 2025
-
Thesis Statement Of A Narrative Essay
Mar 31, 2025
-
Ionic Compounds Dissociate In Water Into
Mar 31, 2025
-
The Ends Of Long Bones Are Called The
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Where On The Periodic Table Are Metals Found . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
