Where Protons And Neutrons Are Located
Muz Play
Mar 30, 2025 · 6 min read
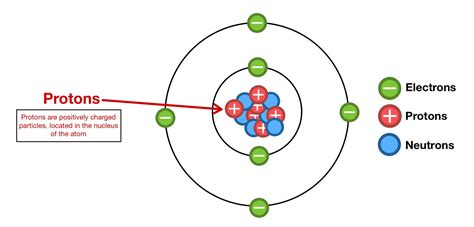
Table of Contents
Where Are Protons and Neutrons Located? A Deep Dive into the Atomic Nucleus
The atom, the fundamental building block of matter, is a fascinating realm of subatomic particles. While electrons whiz around the atom's periphery, creating a diffuse electron cloud, protons and neutrons reside much closer to the atom’s center – the nucleus. Understanding their precise location and the forces that govern their behavior is crucial to grasping the essence of chemistry and physics. This article delves deep into the atomic nucleus, exploring the location, properties, and interactions of protons and neutrons.
The Atomic Nucleus: A Tiny, Dense Powerhouse
The nucleus, a tiny, incredibly dense region at the heart of the atom, houses the majority of the atom's mass. It's here that we find the protons and neutrons, collectively known as nucleons. This nucleus is unimaginably small; if an atom were scaled up to the size of a football stadium, the nucleus would be about the size of a pea in the center. Despite its minuscule size, the nucleus dictates the atom's identity and its behavior in chemical reactions.
The Strong Nuclear Force: Glue of the Nucleus
Holding the nucleus together against the immense repulsive electromagnetic force between positively charged protons is the strong nuclear force. This force is one of the four fundamental forces in nature, and it's incredibly powerful at short distances—the distances within the nucleus. The strong nuclear force is much stronger than the electromagnetic force, but it acts only over extremely short ranges. This explains why protons, despite their mutual repulsion, are held tightly together within the confines of the nucleus. The presence of neutrons is crucial for this stability; they contribute to the strong nuclear force without adding to the electrostatic repulsion.
Protons: The Defining Characteristic of an Element
Protons are positively charged particles. The number of protons in an atom's nucleus, its atomic number, defines the element. For example, an atom with one proton is hydrogen, an atom with six protons is carbon, and an atom with 92 protons is uranium. This number is unique and unchanging for each element; it's what distinguishes one element from another on the periodic table. The positive charge of the protons is balanced by the negative charge of the electrons orbiting the nucleus, making a neutral atom.
Proton Properties: Mass, Charge, and Spin
- Mass: Protons have a mass of approximately 1.6726 × 10⁻²⁷ kg, which is roughly 1836 times the mass of an electron.
- Charge: Protons carry a single positive charge (+1e), which is equal in magnitude but opposite in sign to the charge of an electron.
- Spin: Protons possess an intrinsic angular momentum called spin, which is a quantum mechanical property. This spin is quantized and can be thought of as a rotation, although it's not a classical rotation.
Neutrons: Stabilizing the Nucleus
Neutrons are electrically neutral particles, meaning they carry no charge. Their presence in the nucleus is crucial for the stability of many atoms. While protons' positive charges repel each other, neutrons help to overcome this repulsion by contributing to the strong nuclear force. They act as a kind of "nuclear glue," strengthening the bonds between protons and preventing the nucleus from falling apart.
Neutron Properties: Mass and Spin
- Mass: Neutrons have a mass slightly larger than that of protons, approximately 1.6749 × 10⁻²⁷ kg.
- Spin: Similar to protons, neutrons also possess a quantized spin.
Isotopes: Variations in Neutron Number
The number of neutrons in an atom's nucleus can vary even for the same element. Atoms of the same element with different numbers of neutrons are called isotopes. Isotopes have the same atomic number (number of protons) but different mass numbers (total number of protons and neutrons). Some isotopes are stable, while others are radioactive, meaning they decay over time, emitting particles or energy. This decay process can involve changes in the number of protons, transforming the element into a different one.
Examples of Isotopes:
- Carbon-12 (¹²C): 6 protons and 6 neutrons. This is the most common and stable isotope of carbon.
- Carbon-14 (¹⁴C): 6 protons and 8 neutrons. This is a radioactive isotope used in carbon dating.
- Hydrogen Isotopes: Hydrogen has three isotopes: protium (¹H, one proton, no neutrons), deuterium (²H, one proton, one neutron), and tritium (³H, one proton, two neutrons). Tritium is radioactive.
Nuclear Models: Understanding Nuclear Structure
Several models help us visualize and understand the structure and behavior of the nucleus. These models aren't perfect representations, but they provide valuable insights into the complex interactions within the nucleus.
The Liquid Drop Model
This model treats the nucleus as a drop of incompressible fluid. The nucleons interact with each other through short-range forces, similar to the way molecules interact in a liquid. This model effectively explains some nuclear properties, like nuclear binding energy and nuclear fission.
The Shell Model
The shell model is analogous to the electronic shell model for atoms. It postulates that nucleons occupy discrete energy levels or shells within the nucleus. This model successfully explains the stability of certain nuclei with specific numbers of protons or neutrons (magic numbers).
The Collective Model
This model combines aspects of the liquid drop and shell models. It accounts for both individual nucleon motion and collective nuclear vibrations and rotations. It’s particularly useful in describing the behavior of nuclei with deformed shapes.
The Importance of Nuclear Location and Structure
The location of protons and neutrons within the nucleus is fundamental to our understanding of matter. The precise arrangement and interaction of these nucleons dictate the properties of different elements and isotopes, driving the diversity we observe in the world around us. Furthermore, understanding nuclear structure is essential for various applications, including:
- Nuclear Energy: Harnessing nuclear energy for power generation relies on our understanding of nuclear reactions and stability.
- Nuclear Medicine: Radioactive isotopes are used in medical imaging and treatment, enabling diagnosis and therapy of various diseases.
- Carbon Dating: Radioactive carbon-14 is employed to determine the age of ancient artifacts and organic materials.
Conclusion: A Microscopic Universe of Interactions
The atomic nucleus, with its tightly packed protons and neutrons, is a fascinating microcosm. The strong nuclear force, the interplay of protons and neutrons, and the existence of isotopes all contribute to the rich diversity and complexity of matter. Continuing research into the structure and behavior of the nucleus remains crucial for advancing our understanding of the universe and developing innovative applications in various fields. From the stability of elements to the potential of nuclear energy, the location and interactions of protons and neutrons remain at the heart of fundamental scientific inquiry. The continued exploration of this microscopic universe promises to unlock further insights into the workings of nature itself.
Latest Posts
Latest Posts
-
The Urinary System Regulates Blood Volume And Pressure By
Apr 01, 2025
-
Genomics Can Be Used In Agriculture To
Apr 01, 2025
-
What Is Used For Measuring Mass
Apr 01, 2025
-
Second Moment Of Inertia Parallel Axis Theorem
Apr 01, 2025
-
Single Displacement Reaction Examples In Real Life
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Where Protons And Neutrons Are Located . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
