Which Compounds Will Dissolve In Water
Muz Play
Apr 06, 2025 · 7 min read
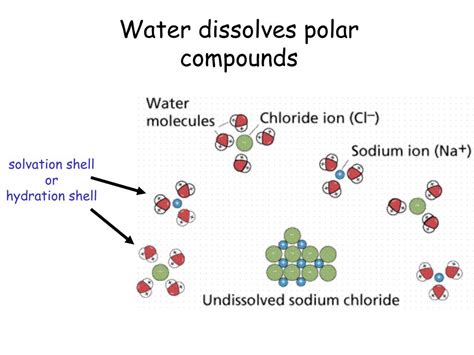
Table of Contents
Which Compounds Dissolve in Water: A Comprehensive Guide
Understanding which compounds dissolve in water is fundamental to chemistry, biology, and numerous other scientific disciplines. Water, as the "universal solvent," plays a crucial role in countless natural and industrial processes. This article delves deep into the factors determining water solubility, exploring different types of compounds and their behavior in aqueous solutions. We'll examine the concepts of polarity, intermolecular forces, and the like-dissolves-like rule to provide a comprehensive understanding of this vital chemical phenomenon.
The Like Dissolves Like Rule: A Cornerstone of Solubility
The cornerstone of predicting solubility is the like dissolves like rule. This principle states that substances with similar polarity tend to dissolve in each other. Polar solvents, like water, effectively dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. Let's break down what this means:
Polarity: The Key to Understanding Solubility
Polarity refers to the distribution of electrical charge within a molecule. In polar molecules, the charge is unevenly distributed, creating a positive and a negative pole (like a magnet). This occurs due to differences in electronegativity between the atoms within the molecule. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. A large difference in electronegativity leads to a polar bond, and a molecule with polar bonds might be polar overall, depending on its geometry.
Water (H₂O) is a prime example of a polar molecule. The oxygen atom is significantly more electronegative than the hydrogen atoms, resulting in a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This polarity is crucial for its solvent properties.
Intermolecular Forces: The Driving Force of Dissolution
The process of dissolution involves the breaking of bonds in the solute and the solvent, and the formation of new interactions between solute and solvent molecules. This is governed by intermolecular forces (IMFs), the attractive forces between molecules. The main types of IMFs include:
-
Hydrogen bonding: A strong type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom in a nearby molecule. Hydrogen bonding is particularly significant in water.
-
Dipole-dipole interactions: These occur between polar molecules due to the attraction between the positive end of one molecule and the negative end of another.
-
London dispersion forces (LDFs): These are weak forces that exist between all molecules, arising from temporary fluctuations in electron distribution. LDFs are particularly important in nonpolar molecules.
For a compound to dissolve in water, the energy gained from the formation of solute-solvent interactions (primarily hydrogen bonds in the case of water) must be greater than the energy required to break the solute-solvent interactions. If the energy balance favors dissolution, the compound will dissolve; otherwise, it will remain undissolved.
Types of Compounds and Their Water Solubility
Let's explore different types of compounds and their solubility in water based on their polarity and intermolecular forces:
1. Ionic Compounds: Generally Soluble in Water
Ionic compounds are composed of positively charged ions (cations) and negatively charged ions (anions) held together by strong electrostatic forces. Water, being a polar molecule, can effectively surround and separate these ions, a process called hydration. The partially negative oxygen atoms of water molecules are attracted to the cations, while the partially positive hydrogen atoms are attracted to the anions. This interaction weakens the ionic bonds and allows the ions to dissolve.
However, not all ionic compounds are equally soluble. The solubility of an ionic compound depends on several factors, including the strength of the ionic bonds and the hydration energy. For example, highly soluble ionic compounds include:
- Sodium chloride (NaCl): Table salt, dissolves readily due to the strong hydration of Na⁺ and Cl⁻ ions.
- Potassium nitrate (KNO₃): Used in fertilizers and explosives, highly soluble in water.
- Many alkali metal salts (Li⁺, Na⁺, K⁺, Rb⁺, Cs⁺) and ammonium salts (NH₄⁺): These cations generally form soluble salts with most anions.
Conversely, some ionic compounds are insoluble or sparingly soluble in water. This often occurs when the lattice energy (the energy required to break the ionic bonds) is very high compared to the hydration energy. Examples include:
- Silver chloride (AgCl): A classic example of a sparingly soluble ionic compound.
- Calcium carbonate (CaCO₃): The main component of limestone, is only slightly soluble.
- Many transition metal salts and heavy metal salts: These often have lower solubility due to stronger ionic bonds and less efficient hydration.
2. Polar Covalent Compounds: Often Soluble in Water
Polar covalent compounds are those where electrons are shared unequally between atoms, resulting in a polar molecule. These molecules can form hydrogen bonds or dipole-dipole interactions with water, leading to solubility. Many sugars, alcohols, and carboxylic acids fall into this category.
Examples of highly soluble polar covalent compounds include:
- Sucrose (table sugar): Dissolves readily in water due to its many hydroxyl (-OH) groups capable of hydrogen bonding.
- Ethanol (C₂H₅OH): A common alcohol that readily mixes with water due to hydrogen bonding.
- Acetic acid (CH₃COOH): The main component of vinegar, soluble due to hydrogen bonding and dipole-dipole interactions.
However, the size and structure of a polar covalent molecule influence its solubility. Very large molecules with many nonpolar parts may exhibit limited solubility, even if they possess some polar groups.
3. Nonpolar Covalent Compounds: Generally Insoluble in Water
Nonpolar covalent compounds involve the equal sharing of electrons between atoms, resulting in nonpolar molecules. These molecules have only weak London dispersion forces and cannot form significant interactions with water. As a result, they are generally insoluble or sparingly soluble in water. This is a direct consequence of the like-dissolves-like rule.
Examples of nonpolar covalent compounds that are insoluble in water include:
- Alkanes (e.g., hexane, octane): These hydrocarbons are completely nonpolar and immiscible with water.
- Oils and fats: These are composed primarily of long hydrocarbon chains and are insoluble in water.
- Many organic solvents (e.g., benzene, toluene): These are typically nonpolar and do not dissolve in water.
4. Gases: Variable Solubility
Gas solubility in water is more complex and influenced by factors such as temperature, pressure, and the nature of the gas. Polar gases, such as ammonia (NH₃) and hydrogen chloride (HCl), dissolve more readily in water than nonpolar gases, like oxygen (O₂) and nitrogen (N₂).
- Polar gases: These dissolve because of their interaction with the polar water molecules.
- Nonpolar gases: Their solubility is lower due to weak interactions with water molecules, and is generally reduced at higher temperatures.
Henry's law describes the solubility of gases in liquids, stating that the solubility of a gas is directly proportional to its partial pressure above the liquid. Increased pressure leads to increased gas solubility.
Factors Affecting Solubility Beyond Polarity
Beyond the like-dissolves-like rule and the types of intermolecular forces, several additional factors influence the solubility of a compound:
-
Temperature: The solubility of most solids in water increases with increasing temperature. This is because increased kinetic energy overcomes the intermolecular forces holding the solid together. However, the solubility of gases in water generally decreases with increasing temperature, as the gas molecules have more kinetic energy and are more likely to escape the solution.
-
Pressure: Pressure primarily affects the solubility of gases in water. Increased pressure forces more gas molecules into the solution, leading to increased solubility (Henry's Law). Pressure has a negligible effect on the solubility of solids and liquids.
-
pH: The pH of the solution can significantly impact the solubility of some compounds. For example, the solubility of many metal hydroxides increases at higher pH (more basic solutions), while the solubility of many metal oxides increases at lower pH (more acidic solutions).
-
Common ion effect: If a solution already contains an ion common to a slightly soluble salt, the solubility of that salt will decrease. This is because the equilibrium of the dissolution reaction shifts to the left, reducing the concentration of dissolved ions.
Conclusion
Predicting whether a compound will dissolve in water involves considering its polarity, the types of intermolecular forces involved, and the interactions between the solute and solvent molecules. The like-dissolves-like rule is a useful guide, but other factors such as temperature, pressure, pH, and the common ion effect can also significantly impact solubility. Understanding these principles is essential in various fields, from designing pharmaceuticals and industrial processes to comprehending biological systems and environmental phenomena. The intricate relationship between solute, solvent, and their interactions paints a fascinating picture of the chemical world.
Latest Posts
Latest Posts
-
Lattice Energy Is An Estimate Of The Bond
Apr 07, 2025
-
What Are The Similarities Between Animal Cells And Plant Cells
Apr 07, 2025
-
Naming Compounds Practice Problems With Answers
Apr 07, 2025
-
Label The Diagram Of The Kidney And Nephron Below
Apr 07, 2025
-
What Does It Mean To Say A Bond Is Polar
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Which Compounds Will Dissolve In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
